- Home Page
- Company Profile
-
Our Products
- Anti Cancer Products
- Antibiotic Drugs
- Pharmaceutical Injection
- Rosinox 60 Mg
- Filgrastim Injection
- Hyaluronidase Injection
- Survanta 8ml Injection
- Viatran 1.5 gm Injection
- Carboplatin Injection
- 5 Flucel 250 Injection
- Pegasta Injection
- Pharmaceutical Injection
- Filgrastim Injection PFS 300 MCG
- Teriparatide Injection
- Neoxal 50 mg Injection
- Pharmaceutical Injection
- Epiruba 50mg Injection
- Zyrop 4000IU Injection
- Encicarb Injection 100mg/2ml
- Vintor 4000 Injection
- Acopacli 100 mg Injection
- Stpase 1500000iu Injection
- Colistimethate Sodium Injection IP 1 Million IU (Xylistin)
- Tazact 4.5 gm injection
- Merocrit 1gm injection
- Bortezomib injection
- Grastim Injection
- Rhoclone 300 Mcg Injection
- Gemita 1400mg Injection
- Viatran 1.5 gm Injection
- Grafeel m30 injection
- Platin 50mg Infusion
- Menopur 75 Iu Injection
- Tigez 50 Mg Injection
- Encicarb 500 Mg Injection
- Myezom 2mg Injection
- Aldesleukin 1.3 mg injection
- Palnox 0.25mg Injection
- Dargen 60 Injection
- Axicabtagene Ciloleucel injection
- Abevmy 100 mg Injection
- Abevmy 400 mg Injection
- Acodamust 100 mg Injection
- Acodocet 20 mg Injection
- Acopacli 30 mg Injection
- Acopacli 260 mg Injection
- Acotrexed 100 mg Injection
- Acotrexed 500 mg Injection
- Acoxal 50 mg Injection
- Acoxal 100 mg Injection
- Acozacit 100 mg Injection
- Actemra 200 mg Injection
- Actemra 80 mg Injection
- Actide 0.05 mg Injection
- Actide 0.1 mg Injection
- Actilyse 20 mg Injection
- Actilyse 50 mg Injection
- Actinocin 0.5 mg Injection
- Actorise 100 mcg Injection
- Actorise 200 mcg Injection
- Adben 100 mg Injection
- Adcarb 150 mg Injection
- Adcarb 450 mg Injection
- Adcov 50 mg Injection
- Adfill 6 mg Injection
- Adoxi 120 mg Injection
- Adoxi 80 mg Injection
- Adpaxil 30 mg Injection
- Adpaxil 100 mg Injection
- Adriamycin 10 mg Injection
- Adriamycin 50 mg Injection
- Adriamycin RTU 10 mg Injection
- Adrib 10 mg Injection
- Adrib 50 mg Injection
- Adricin 10 mg Injection
- Adricin 50 mg Injection
- Adrim 10 mg Injection
- Adrim 50 mg Injection
- Adrisome 20 mg Injection
- Adronex RD 10 mg Injection
- Adronex RD 50 mg Injection
- Adrosal 50 mg Injection
- Adside 100 mg Injection
- Adsunib 12.5 mg Capsule
- Adsunib 25 mg Capsule
- Adsunib 50 mg Capsule
- Advamab 100 mg Injection
- Advamab 400 mg Injection
- Albucel 10 gm/50 ml Injection
- Albucel 20 gm/100 ml Injection
- Albumax Neo 20% 100 ml Injection
- Alimta 100 mg Injection
- Alimta 500 mg Injection
- Alkacel PGF 50 mg Injection
- Alrubicin 100 mg Injection
- Alrubicin 100 mg Injection
- Alrubicin 10 mg Injection
- Alrubicin 50 mg Injection
- Arpimune ME 100 mg Oral Suspension
- Arsenox Injection
- Altaxel NOVA 100 mg Injection
- Altaxel NOVA 260 mg Injection
- Arsitri 10 mg Injection
- Altaxel NOVA 30 mg Injection
- Avangio 100 mg Injection
- Avangio 400 mg Injection
- Avastimab 100 mg Injection
- Avastimab 400 mg Injection
- Avastin 100 mg Injection
- Avastin 400 mg Injection
- Ambisome 50 mg Injection
- Azacite 100 mg Injection
- Amfosted 500 mg Injection
- Azactiv 100 mg Injection
- Azacytin 100 mg Injection
- Amgicin 200 mg Injection
- Azadine 100 mg Injection
- Amgicin 1000 mg Injection
- Amitant 150 mg Injection
- Azafab 100 mg Injection
- Azafect 100 mg Injection
- Azakem 100 mg Injection
- Azalive 100 mg Injection
- Azaplast 100 mg Injection
- Azashine 100 mg Injection
- Azatend 100 mg Injection
- Azatirel Injection
- Azishil 100 mg Injection
- Azzure 100 mg Injection
- Amphocel 50 mg Injection
- Ancount 300 mcg Injection
- Aprecap 150 mg Injection
- Arasid 1000 mg Injection
- Arasid 100 mg Injection
- Arasid 500 mg Injection
- Arbitus 500 mg Tablet
- Bandrone 6 mg Injection
- Bdnab 100 mg Injection
- Bdolenic 4 mg Injection
- Bdsure 11.25 mg Injection
- Bdsure 22.5 mg Injection
- Bdsure 3.75 mg Injection
- Bd taxel 100 mg Injection
- Bd taxel 260 mg Injection
- Bd taxel 300 mg Injection
- Bleocip 15IU Injection
- BLEOZ 15IU INJECTION
- Bonimet 6 mg injection
- Bevetex 100 mg Injection
- Bortecad 2 mg Injection
- Bortenat 2 mg Injection
- Bemustin 100 mg Injection
- Bendamax 100 mg Injection
- Bendit 100 mg Injection
- Benzz 100 mg Injection
- Benzz 180 mg Injection
- Benzz 45 mg Injection
- Berirab P 300 IU
- Bortenat 3.5 mg Injection
- Bortero 2 mg Injection
- Bortether 2 mg Injection
- Bortetrust 2 mg Injection
- Bortezom 2 mg Injection
- Bortiad 2 mg Injection
- Bortirel 3.5 mg Injection
- Bortrac 2 mg Injection
- Borviz 2 mg Injection
- Borviz 2.5 Injection
- Borviz 3.5 mg Injection
- Bryxta 100 mg Injection
- Besponsa 1 mg Injection
- Bryxta 300 mg Injection
- Bryxta 400 Injection
- Bucelon 60 mg Injection
- Betaferon 250 mcg Injection
- Bufatas IV Injection
- Bevac Vaccine (Adult) 1 ml
- Busarlin 7 mg Injection
- Buselin 0.5 mg Injection
- Buselin 7 mg Injection
- Bevacirel 100 mg Injection
- Bevacirel 400 mg Injection
- Busulmax 60 Injection
- Cabaxan Injection
- Cabazirix 60 mg Injection
- Bevarest 100 mg Injection
- Bevarest 400 mg Injection
- Cabtana 60 mg Injection
- Bevaro 100 mg Injection
- Cadria 10 mg Injection
- Bevaro 400 mg Injection
- Bevatas 100 mg Injection
- Caelyx 2 mg Infusion
- Calcium Folinate 50 mg Injection
- Bevatas 400 mg Solution for Infusion
- Bevazza 100 mg Injection
- Bevazza 400 mg Injection
- Bevetex 300 mg Injection
- Campath 30 mg Injection
- Campto 40 mg Injection
- Campto 100 mg Injection
- Canmab 150 mg Injection
- Canmab 440 mg Injection
- Bevicra 100 mg Injection
- Bevicra 400 mg Injection
- Carbokem Nova 150 mg Injection
- Carbokem Nova 450 mg Injection
- Bharglob 16.5% Injection
- Carbomax 150 mg Injection
- Carbomax 450 mg Injection
- Carbopa 150 mg Injection
- Carbopa 450 mg Injection
- carboplatin 150 mg injection
- Carboplatin 450 mg Injection
- Carboteen 150 mg Injection
- Carboteen 450 mg Injection
- Carbotero 150 mg Injection
- Carbotero 450 mg Injection
- Biceltis 440 mg Injection
- Carbozar 150 mg Injection
- Bimode 100 mg Injection
- Carbozar 450 mg Injection
- Carfilnat 60 mg Injection
- Biobin 100 mg Injection
- Carmuther 100 mg Injection
- Carnumax 100 Injection
- Carustine 100 mg Injection
- Carzomib 60 mg Injection
- Cazat Injection
- Cdoxin 50 mg Injection
- Celcarb 150 mg Injection
- Celcarb 450 mg Injection
- Celdach 50 mg Injection
- Celdach 100 mg Injection
- Celdaz 100 mg Injection
- Biobin 500 mg Injection
- Celdaz 200 mg Injection
- Celdaz 500 mg Injection
- Celgem 200 mg Injection
- Celgem 1000 mg Injection
- Cellcept 1 gm suspension
- Biocarb 150 mg Injection
- Celofos 1 gm Injection
- Celofos 2 gm Injection
- Celoside 100 mg Injection
- Celplat 10 mg Injection
- Celplat 50 mg Injection
- Celrixafor 24 mg Injection
- Celtax 30 mg Injection
- Celtax 300 mg Injection
- Celtere 20 mg Injection
- Celtere 80 mg Injection
- Celtere 120 mg Injection
- Celvestrant 250 mg Injection
- Celzar 200 mg Injection
- Biocarb 450 mg Injection
- Cetrolix 0.25 mg Injection
- Biocarb 450 mg Injection
- Cetrolix 0.25 mg PFS
- Cfara Injection
- Biocristin AQ 1 mg Injection
- Chemoblast 10 mg Injection
- Cismax 10 mg Injection
- Cismax 50 mg Injection
- Cisteen 10 mg Injection
- Cistero 10 mg Injection
- Cistero 50 mg Injection
- Citafine 1 gm Injection
- Citafine 200 mg Injection
- Citafine 1400 mg Injection
- Cizcan 10 mg Injection
- Cizcan 50 mg Injection
- Cizumab 100 mg Injection
- Cizumab 400 mg Injection
- Cladrim 10 mg Injection
- Colstim 300 mcg Injection
- ConsIUm 100 mg Injection
- Biocure 2 mg Injection
- Coriosurge XP 5000 IU Injection
- Coriosurge XP 10000 IU Injection
- Cosmegen 0.5 mg Injection
- Biodronate 30 mg Injection
- Cresemba 200 mg Injection
- Cresp 25 mcg Injection
- Cresp 40 mcg PFS
- Cresp 60 mcg Injection
- Cresp 100 mcg PFS
- Cresp 200 mcg PFS
- Biodronate 60 mg Injection
- Cresp 500 mcg PFS
- Ctx gls 1 gm Injection
- Ctx gls 200 mg Injection
- Ctx gls 500 mg Injection
- Cyclocel 200 mg Injection
- Cyclocel 500 mg Injection
- Cyclocel 1000 mg Injection
- Cycloxan 200 mg Injection
- Cycloxan 500 mg Injection
- Cycloxan 1000 mg Injection
- Biodronate 90 mg Injection
- Cyebulin Injection
- Cyphos 1 gm Injection
- Cyphos 200 mg Injection
- Cyphos 500 mg Injection
- Cyramza 100 mg Injection
- Cyramza 500 mg Injection
- Biogem 200 mg Injection
- Cytalon 100 mg Injection
- Cytalon 500 mg Injection
- Cytalon 1000 mg Injection
- Cytaneon 1000 mg Injection
- Cytarabine 1000 mg Injection
- Cytarine 1 gm Injection
- Cytarine 100 mg Injection
- Cytarine 500 mg Injection
- Cytax 30 mg Injection
- Cytax 100 mg Injection
- Cytax 260 mg Injection
- Cytax 300 mg Injection
- Cytax NAB 100 mg Injection
- Cytoblastin 10 mg Injection
- Cytocarb 150 mg Injection
- Cytocarb 450 mg Injection
- Cytocristin 1 mg Injection
- Biomab EGFR 50 mg Injection
- Bionase 10000 IU Injection
- Cytocristin Aqueous 1 mg Injection
- Bionase 5000 IU Injection
- Biovac A Vaccine
- Cytogem 200 mg Injection
- Cytogem 1000 mg Injection
- Cytomab 100 mg Injection
- Cytomab 500 mg Injection
- Cytoplatin 10 mg Injection
- Cytoplatin 50 mg Injection
- Cytosar 100 mg Injection
- Cytosar 500 mg Injection
- Cytosar 1000 mg Injection
- Cytowel 500 mg Injection
- Cytowel 1000 mg Injection
- Dabaz 200 mg Injection
- Dabaz 500 mg Injection
- Dacilon 0.5 mg Injection
- Biovorin 50 mg Injection
- Dacmozen 0.5 mg Injection
- Bleocel 15IU Injection
- Dacotin 50 mg Injection
- Dacotin 100 mg Injection
- Dactinoget 0.5 mg Injection
- Darbecure 25 mcg PFS
- Bleochem 15 IU Injection
- Darbecure 40 mcg PFS
- Bleocin 15IU Injection
- Darzalex 100 mg Injection
- Darzalex 400 mg Injection
- Dauneon 20 mg Injection
- Daunocyte 20 mg Injection
- Daunorubitec 20 mg Injection
- Daunotec 20 mg Injection
- Daxotel 20 mg Injection
- Daxotel 80 mg Injection
- Daxotel 120 mg Injection
- Decapeptyl 0.1 mg Injection
- Decapeptyl Depot Injection
- Decarb 200 mg Injection
- Decarb 500 mg Injection
- Decintas 30 mg Injection
- Decintas 50 mg Injection
- Decitas 30 mg Injection
- Decitas 50 mg Injection
- Decitex 30 mg Injection
- Decitex 50 mg Injection
- Deczuba 50 mg Injection
- Degalix 80 mg Injection
- Degalix 120 mg Injection
- Nanoxel 30 mg Injection
- Degapride 80 mg Injection
- Degapride 120 mg Injection
- Degatide 80 mg Injection
- Degatide 120 mg Injection
- Deghor 80 mg Injection
- Deghor 120 mg Injection
- Degrinta 80 mg Injection
- Degrinta 120 mg Injection
- Deninta SC 60 mg PFS
- Denu 60 mg PFS
- Nanoxel 30 mg Injection
- Naprodox 10 mg Injection
- Naprodox 50 mg Injection
- Natdecita 50 mg Injection
- Dobixin 10 mg Injection
- Dobixin 50 mg Injection
- Docax 20 mg Injection
- Docax 80 mg Injection
- Docax 120 mg Injection
- Doceaqualip 20 mg Injection
- Doceaqualip 80 mg Injection
- Docefect RTU 20 mg Injection
- Docefect RTU 80 mg Injection
- Docel 120 mg Injection
- Docemax 20 mg Injection
- Docetax 20 mg Injection
- Docetax 80 mg Injection
- Docetax 120 mg Injection
- Docetec 20 mg Injection
- Docetec 80 mg Injection
- Docetec 120 mg Injection
- Docetere 20 mg Injection
- Docetere 80 mg Injection
- Docetere 120 mg Injection
- Docetero 20 mg Injection
- Docetero 80 mg Injection
- Docetero 120 mg Injection
- Natdox-LP Injection
- Docetrust 20 mg Injection
- Natzold 5 mg infusion
- Docetrust 80 mg Injection
- Docetrust 120 mg Injection
- Navelbine 10 mg Injection
- Docezap 20 mg Injection
- Navelbine 50 mg Injection
- Doxilyd 10 mg Injection
- Doxilyd 50 mg Injection
- Doxocare 50 mg Injection
- Doxomed 10 mg Injection
- Doxorex 10 mg Injection
- Doxorex 50 mg Injection
- Doxoruba 10 mg Injection
- Doxoruba 50 mg Injection
- Nab Altaxel 100 mg Injection
- Nab Celtax 100 mg Injection
- Doxorubin 10 mg Injection
- Doxorubin 50 mg Injection
- Doxotero 10 mg Injection
- Doxotero 50 mg Injection
- Doxulip 20 mg Injection
- Doxulip 50 mg Injection
- Doxutec 10 mg Injection
- Doxutec 50 mg Injection
- Nab Celtax 100 mg Injection
- Dutaxel 30 mg Injection
- Dutaxel 100 mg Injection
- Dutaxel 260 mg Injection
- Ebunat 0.5 mg Injection
- Eleftha 150 mg Injection
- Eleftha 375 mg Injection
- Eleftha 440 mg Injection
- Eligard 7.5 mg Injection
- Eligard Depot 22.5 mg Injection
- Eligard Depot 45 mg Injection
- Embremma 0.5 mg Injection
- Emend 150 mg Injection
- Emetant 150 mg Injection
- Emgrast 300 mcg Injection
- Emgrast m 500 mcg Injection
- Emtreo 5 mg Injection
- Emtux 100 mg Injection
- Emtux 500 mg Injection
- Enbrel 50 mg Injection
- Encicarb 1 k Injection
- Nab Mitotax 100 mg Injection
- Encicarb 500 mg Injection
- Endamust 100 mg Injection
- Endokine 300 mcg PFS
- Endoxan 200 mg Injection
- Endoxan 1000 mg Injection
- Endoxan N 1 gm Injection
- Endoxan N 200 mg Injection
- Endoxan N 500 mg Injection
- Epichlor 10 mg Injection
- Epichlor 50 mg Injection
- Epiget 10 mg Injection
- Epiget 50 mg Injection
- Epineon 10 mg Injection
- Epineon 50 mg Injection
- Epirubitec 10 mg Injection
- Epirubitec 50 mg Injection
- Epitaz 10 mg Injection
- Epitaz 50 mg Injection
- Epitaz 100 mg Injection
- Epitero 50 mg Injection
- Epithra 10 mg Injection
- Epithra 50 mg Injection
- Epithra 100 mg Injection
- Epixtra 10 mg Injection
- Epixtra 50 mg Injection
- Epofit 2000 IU Injection
- Epofit 4000 IU PFS
- Epofit 5000 IU PFS
- Epofit 6000 IU PFS
- Epofit 10000 IU PFS
- Epofit 20000 IU Vial
- Eribilin Injection
- Erubin 10 mg Injection
- Erubin 50 mg Injection
- Erykine 10000 IU Injection
- Erypeg 30 mcg PFS
- Erypeg 50 mcg PFS
- Erypeg 75 mcg PFS
- Erypeg 100 mcg PFS
- Esentra 60 mg PFS
- Esentra 120 mg Injection
- Espogen 10000 IU Injection
- Etolon 100 mg Injection
- Etoplast 100 mg Injection
- Etosid 100 mg Injection
- Etovel 100 mg Injection
- Eurolide 1 mg Injection
- Eurolide 4 mg Injection
- Eurolide Depot 3.75 mg Injection
- Eutropin 4IU Injection
- Evaira 0.5 mg Injection
- Evaparin 40 mg PFS
- Evaparin 300 mg Cartridge
- Exemptia 40 mg PFS
- Eylea 40 mg Injection
- Factocel VIII 250 IU Injection
- Fanhdi 250 IU Injection
- Fanhdi 500 IU Injection
- Farabine 20 mg Injection
- Farmorubicin RD 10 mg Injection
- Farmorubicin RD 50 mg Injection
- Farmorubicin RTU 10 mg Injection
- Farmorubicin RTU 50 mg Injection
- Faslodex 250 mg Injection
- Faslomax 250 mg Injection
- Fasnorm 250 mg Injection
- Fastograf 300 mcg Injection
- Fibrogen I Injection
- Filcad 300 mcg Injection
- Filcad P 6 mg PFS
- Filneon 300 mcg Injection
- Firmagon 80 mg Injection
- Firmagon 120 mg Injection
- Flexbumin 20 gm100ml (BAG) Infusion
- Fluarix Tetra 20222023 NH
- Fludacel 50 mg Injection
- Fludara 50 mg Injection
- Fludarither 50 mg Injection `
- Fludocyte 50 mg Injection
- Fluracil 250 mg Injection
- Fluracil 500 mg Injection
- Folisurge 75 IU PFS
- Folisurge 150 IU PFS
- Folisurge 225IU PFS
- Folisurge 300IU PFS
- Folisurge 900IU Cartridge
- Folisurge 900IU Pen
- Folisurge1200 IU Injection
- Fosa 150 mg Injection
- Neoben 50 mg Injection
- Fosaport 150 mg Injection
- Fosapp 150 mg Injection
- Fosapretero 150 mg Injection
- Fosaran 150 mg Injection
- Fosfa 1 gm Injection
- Fosfa 2 gm Injection
- Neoctide 0.1 mg Injection
- Fosup 150 mg Injection
- Fragmin 2500 IU Injection
- Fragmin 5000 IU Injection
- Fragmin 10000 IU Injection
- Frastim 300 mcg Injection
- Neoctide 0.05 mg Injection
- Fulvenat 250 mg Injection
- Neomib 2 mg Injection
- Fulveser 250 mg Injection
- Fulvether 250 mg Injection
- Fulvetraz 250 mg Injection
- Fulvidax 250 mg Injection
- Fulviglen 250 mg PFS
- Fulvira 250 mg Injection
- Fulzos 250 mg PFS
- Fuvestrol 250 mg Injection
- Fytosid 100 mg Injection
- Gardasil Vaccine
- Neoxal 100 mg Injection
- Neukine 150 mcg Injection
- Neukine 300 mcg Injection
- Gembin 200 mg Injection
- Gembin 1000 mg Injection
- Neukine 300 mcg Vial
- Gemchem 200 mg Injection
- Gemchem 1000 mg Injection
- Gemchem 1400 mg Injection
- Gemcite 1 gm Injection
- Neupeg 6 mg Injection
- Gemcite 200 mg Injection
- Gemdronic 5 mg Injection
- Gemfect 1 gm Injection
- Gemfect 200 mg Injection
- Neuzumab 150 mg Injection
- Gemget 1 gm Injection
- Neuzumab 440 mg Injection
- Gemget 200 mg Injection
- Gemibine 1.4 gm Injection
- Gemibine 200 mg Injection
- Gemibine 1000 mg Injection
- Gemita 1 gm Injection
- Gemita 1.4 gm Injection
- Gemizan 1 gm Injection
- Gempower 1 gm Injection
- Gempower 200 mg Injection
- Gemtaz 1 gm Injection
- Gemtaz 200 mg Injection
- Gemtero 1 gm Injection
- Gemtero 200 mg Injection
- Nexben 100 mg Injection
- Gemtide 600 mcg Injection
- Giopem 100 mg Injection
- Giopem 500 mg Injection
- Glenstime PEG 6 mg PFS
- Glerelin 22.5 mg Injection
- Globucel 5 gm100 ml Injection
- Globucel 10 gm100 ml Injection
- Globucel SC Injection
- Gonapeptyl 3.75 mg Injection
- Grafeel 300 mcg PFS
- Grastim 300 mcg PFS
- Grastim 300 mcg Vial
- Gros 30 mg Injection
- Gros 100 mg Injection
- Gros 260 mg Injection
- Gynarich Injection
- Halaven 1 mg Injection
- Hamsyl 3750 IU Injection
- Herceptin Infusion
- Herclon 440 mg Injection
- Herclon Injection
- Herhope 440 mg Injection
- Hermab 150 mg Injection
- Hermab 440 mg Injection
- Hersima 150 mg Injection
- Hersima 440 mg Injection
- Herticad 440 mg Injection
- Hertraz 150 mg Injection
- Hertraz 440 mg Injection
- Hervycta 150 mg Injection
- Hervycta 440 mg Injection
- Holoxan with uromitexan 1 gm Injection
- Holoxan with uromitexan 2 gm Injection
- Human Albumin 20 gm100 ml Injection
- Humog 150 HP Injection
- I-dox 50 mg Injection
- Idromet 6 mg Injection
- Ifex-m 1 gm Injection
- Ifoneon 1 gm Injection
- Nitrol 20 mg Injection
- Ifopar 1 gm Injection
- Nitsum 100 mg Injection
- Ifopar 2 gm Injection
- Ikgdar 100 mg Injection
- Ikgdar 500 mg Injection
- Novotaxel 120 mg Injection
- Novotaxel 20 mg Injection
- Novotaxel 80 mg Injection
- Imfinzi 120 mg Injection
- Imfinzi 500 mg Injection
- Imtus 40 mg Injection
- Imtus 100 mg Injection
- Imumax 300 mcg Injection
- Imupeg 6 mg Injection
- Innocar 150 mg Injection
- Innocar 450 mg Injection
- Innogem 200 mg Injection
- Innogem 1000 mg Injection
- Innotubilin 20 mg Injection
- Innotubilin 80 mg Injection
- Instantino 0.25 Injection
- Intacept 25 mg PFS
- Intacept 50 mg PFS
- Intaxel 30 mg Injection
- Intaxel 100 mg Injection
- Intaxel 260 mg Injection
- Intensic 40 mg Injection
- Intensic 100 mg Injection
- Ipoget whith mesna 1 gm Injection
- Irinotel 40 mg Injection
- Irinotel 100 mg Injection
- Iritero 40 mg Injection
- Iritero 100 mg Injection
- Irnocam 40 mg Injection
- Irnocam 100 mg Injection
- Irnocel 100 mg Injection
- Isoxan 1 gm Injection
- Ivnex 5 gm Injection
- Nudoxa 50 mg Injection
- Jevatax 60 mg Injection
- Jevtana Injection
- Kabanat Injection
- Kabipem 100 mg Injection
- Kabipem 500 mg Injection
- Nufil safe 300 mcg Injection
- Kadcyla 100 mg Injection
- Kadcyla 160 mg Injection
- Kemocarb 150 mg Injection
- Kemocarb 450 mg Injection
- Kemoplat 10 mg Injection
- Kemoplat 50 mg Injection
- Keytruda 100 mg Injection
- Krabeva 100 mg Injection
- Krabeva 400 mg Injection
- Kyprolis 60 mg Injection
- Lagicad 5000 IU Injection
- Lagicad 10000 IU Injection
- Lagipeg 3750 IU Injection
- Nufil sfs 300 mcg Injection
- L-ASAP Injection
- Lastet 100 mg Injection
- Octotide 0.05 mg Injection
- Octotide 0.1 mg Injection
- Leprol 22.5 mg Injection
- Leuben 100 mg Injection
- Leucorin 15 mg Injection
- Leucorin 50 mg Injection
- Leucovorin calcium 15 mg Injection
- Leucovorin calcium 50 mg Injection
- Leuget 50 mg Injection
- Leugard 22.5 mg Injection
- Leupo 11.25 mg Injection
- Leupo 22.5 mg Injection
- Leuprosta Depot 11.25 mg Injection
- Leuprosta Depot 22.5 mg Injection
- Leutero 50 mg Injection
- Lippod 20 mg Injection
- Lippod 50 mg Injection
- Lonopin MD 300mg Injection
- Lupifil 300 mcg Injection
- Lupifil P 6 mg Injection
- Lupiximab 100 mg Injection
- Lupiximab 500 mg Injection
- Lupride 1 mg Injection
- Lupride 4 mg Injection
- Lupride Depot 3.75 mg Injection
- Lupride Depot 11.25 mg Injection
- Lupride Depot 22.5 mg Injection
- Luprodex 3.75 mg Injection
- Luprodex 3M 11.25 mg Injection
- Octride 0.1 mg Injection
- Luprodex Depot 11.25 mg Injection
- Luprodex Depot 22.5 mg Injection
- Luprorin 4 mg Injection
- Luprotas 11.25 mg Injection
- Luprotas 22.5 mg Injection
- Luprova 3.75 mg Injection
- Luprova 11.25 mg Injection
- Luprova 22.5 mg Injection
- Lutrate 3.75 mg Injection
- Lutrate 22.5 mg Injection
- Lyoble 15 IU Injection
- Lyoble Injection
- Lyomit 10 mg Injection
- Lyphidox 50 mg Injection
- Maball 100 mg Injection
- Maball 500 mg Injection
- Mabtas 100 mg Injection
- Mabtas 500 mg Injection
- Mabtas N 500 mg Injection
- Mabtas RA 500 mg Injection
- Mabtas T 100 mg Injection
- Mabtas T 500 mg Injection
- Mabura 40 mg PFS
- Mabvinra 40 mg PFS
- Octride Depot 20 mg Injection
- Octride Depot 30 mg Injection
- Materna Depot 3.75 mg Injection
- Maxtorin 100 mg Injection
- Mds plus 100 mg Injection
- Megaplat 50 mg Injection
- Megaplat 100 mg Injection
- Megval 50 mg Injection
- Mejorasp 3750 IU Injection
- Melfalax 50 mg Injection
- Melphather 50 mg Injection
- Menotas 75 IU Injection
- Menotas 150 IU Injection
- Menotas HP 75 IU Injection
- Menotas HP 150 IU Injection
- Menotas XP 1200 IU Injection
- Merex 500 mg Injection
- Merex 1000 mg Injection
- Mesna 200 mg Injection
- Metodox 10 mg Injection
- Metodox 50 mg Injection
- Mircera 50 mcg Injection
- Mircera 75 mcg Injection
- Mircera 100 mcg Injection
- Mistabron 200 mg solution for Inhalation
- Mito 10 mg Injection
- Mitobulin 0.5 mg Injection
- Mitocin 10 mg Injection
- Mitomycin 2 mg Injection
- Mitomycin 10 mg Injection
- Mitomycin 40 mg Injection
- Mitomycin C 2 mg Injection
- Mitomycin C 10 mg Injection
- Mitotax 30 mg Injection
- Mitotax 100 mg Injection
- Mitotax 250 mg Injection
- Mitotax 300 mg Injection
- Okeron 0.1 mg Injection
- Mostim 24 mg Injection
- Mozifor 24 mg Injection
- Myaza 100 mg Injection
- Myezom 2 mg Injection
- Myezom 3.5 mg Injection
- Mygem 1 gm Injection
- Mygem 200 mg Injection
- Mymust 100 mg Injection
- Myzomib 2 mg Injection
- Otide 0.1 mg Injection
- Otide 0.05 mg Injection
- Oxa 50 mg Injection
- Oxa 100 mg Injection
- Oxalimax 100 mg Injection
- Oxaliptin 50 mg Injection
- Oxaliptin 100 mg Injection
- Oxaltero 50 mg Injection
- Oxashil 100 mg Injection
- Oxifect 50mg Injection
- Oxifect 100 mg Injection
- Onco BCG 40 mg Injection
- Oxiplat 50 mg Injection
- Oxiplat 100 mg Injection
- Oxitan 50 mg Injection
- Oxitan 100 mg Injection
- Oxitoz 50 mg Injection
- Oxitoz 100 mg Injection
- Oxoplan 50 mg Injection
- Oxoplan 100 mg Injection
- Oxpla 50 mg Injection
- Oxpla 100 mg Injection
- Oxyrea 50 mg Injection
- Oxyrea 100 mg Injection
- Pacliall 100 mg Injection
- Pacliaqualip 30 mg Injection
- Pacliaqualip 100 mg Injection
- Paclicad 100 mg injection
- Paclicad N Injection
- Oncofluor 250 mg Injection
- Paclimin 100 mg Injection
- Pacliget 260 mg Injection
- Paclistar 30 mg Injection
- Paclistar 100 mg Injection
- Paclistar 260 mg Injection
- Paclitax 30 mg Injection
- Oncofluor 500 mg Injection
- Paclitax 100 mg Injection
- Paclitax 260 mg Injection
- Paclitax 300 mg Injection
- Paclitax NAB 100 mg Injection
- Paclitec 30 mg Injection
- Paclitec 100 mg Injection
- Paclitec 260 mg Injection
- Oncogem 1.4 gm Injection
- Oncogem 200 mg Injection
- Paclitero 30 mg Injection
- Paclitero 100 mg Injection
- Paclitero 260 mg Injection
- Pacshil 30 mg Injection
- Pacshil 100 mg Injection
- Pacshil 260 mg Injection
- Palnox 0.25 mg Injection
- Palzen 0.25 mg Injection
- Oncomide 1000 mg Injection
- Oncomide 1000 mg Injection
- Oncomide 200 mg Injection
- Oncotron 20 mg Injection
- Onvinc 1 mg Injection
- Opdyta 40 mg Injection
- Opdyta 100 mg Injection
- Oplatin 50 mg Injection
- Oplatin 100 mg Injection
- Ortez 2 mg Injection
- Pamidria 60 mg Injection
- Pamidria 90 mg Injection
- Pamifect 100 mg Injection
- Pamifect 500 mg Injection
- Pamorelin LA 3.75 mg Injection
- Pamorelin LA 11.25 mg Injection
- Pamorelin LA 22.5 mg Injection
- Pasetron 0.25 Injection
- Pataxel 30 mg Injection
- Ostemet 4 mg Injection
- Acodocet 80 mg Injection
- Pataxel 100 mg Injection
- Pataxel 260 mg Injection
- Peg Frastim 6 mg Injection
- Peg Grafeel 6 mg PFS
- Pegadria 20 mg Injection
- Pegadria 50 mg Injection
- Pegasta 6 mg Injection
- Pegasta Injection
- pegex 6 mg injection
- Pegfeel 6 mg PFS
- Pegg Trust 6 mg Injection
- Peg-Ginase 3750 IU Injection
- Peg L-Aspatero 3750 IU Injection
- Peg Religrast 6 mg Injection
- Pegihep 80 mcg Injection
- Pegihep 100 mcg Injection
- Pegihep 120 mcg Injection
- Pemcure 100 mg Injection
- Pemcure 500 mg Injection
- Pemeplast 100 mg Injection
- Pemeta 100 mg Injection
- Pemeta 500 mg Injection
- Pemetero 100 mg Injection
- Pemetero 500 mg Injection
- Pemexar 100 mg Injection
- Pemexar 500 mg Injection
- Pemgem 100 mg Injection
- Pemgem 500 mg Injection
- Pemmet 100 mg Injection
- Pemmet 500 Injection
- Pemnat 100 mg Injection
- Pemnat 500 mg Injection
- Pempro 100 mg Injection
- Pempro 500 mg Injection
- Pemshil 100 mg Injection
- Pemshil 500 mg Injection
- Perjeta 420 mg Injection
- Bortetrust 2 Injection
- Cabapan Injection
- Petaxel 30 mg Injection
- Petaxel 100 mg Injection
- Petaxel 260 mg Injection
- Pexetrust 500 mg Injection
- Pexitaz 100 mg Injection
- Pexitaz 500 mg Injection
- Pexotra 100 mg Injection
- Pexotra 500 mg Injection
- Phoxelon 1000 mg Injection
- Picasa Injection
- Picasa Suspension
- Plamumab 40 mg PFS
- Plasbumin 20% Injection
- Platinex 10 mg Injection
- Platinex 50 mg Infusion
- Posid 100 mg Injection
- Prexx 500 mg Injection
- Procabazi Injection
- Prolia 60 mg PFS
- Proteoz 2 mg Injection
- Pro-throm 250 IU Injection
- Purplz 100 mg Injection
- Rasbelon 1.5 mg Injection
- Rasburnat 1.5 mg Injection
- Rasby 1.5 mg Injection
- Rascase 1.5 mg Injection
- Razumab Injection
- Reditux 100 mg Injection
- Reditux 500 mg Injection
- Reditux 600 mg Injection
- Reditux RA 500 mg Injection
- Relbovin 50 Injection
- Religrast 300 mcg Injection
- Relitrexed 100 mg Injection
- Relitrexed 500 mg Injection
- Remicade Injection
- Renocel 2000 IU PFS
- Renocel 4000 IU PFS
- Renocel 6000 IU PFS
- Renocel 10000 IU PFS
- Renocrit 4000 IU PFS
- Renocrit 10000 IU PFS
- Repatha 140 mg Injection
- Ribomustin 100 mg Injection
- Rinotec 100 mg Injection
- Ristova 100 mg Injection
- Ristova 500 mg Injection
- Ritucad 100 mg Injection
- Ritucad 500 mg Injection
- Ritulasta 100 mg Injection
- Ritulasta 500 mg Injection
- Rituxem 100 mg Injection
- Rituxem 500 mg Injection
- Rituxirel 100 mg Injection
- Rituxirel 500 mg Injection
- Rituxirel RN 500 mg Injection
- Rokfos Infusion
- Romiset 250 mcg injection
- Romy 250 mcg Injection
- Romy 500 mcg Injection
- Rosinox 40 mg Injection
- Rozel 60 mg PFS
- Rubilon 10 mg Injection
- Rubilon 50 mg Injection
- Rubilon 100 mg Injection
- Sandostatin 0.1 mg Injection
- Sandostatin 0.05 mg Injection
- Sandostatin LAR 20 mg Injection
- Sandostatin LAR 30 mg Injection
- Scapho 150 mg Injection
- Shilgem 1 gm Injection
- Shilstine 50 mg Injection
- Sirotem Injection
- Solfredoc 20 mg Injection
- Solfredoc 80 mg Injection
- Solfredoc 120 mg Injection
- Soxplat 50 mg Injection
- Soxplat 100 mg Injection
- Stemfor 24 mg Injection
- Stimufol HP 75 IU Injection
- Stimufol HP 150 IU Injection
- Stoplos One 5 mg100ml Infusion
- Strantas 250 mg Injection
- Stricarb 150 mg Injection
- Stricarb 450 mg Injection
- Stridox 50 mg Injection
- Stritin 10 mg Injection
- Stritin 50 mg Injection
- Syndyma 100 mg Injection
- Syndyma 400 mg Injection
- Taxeleon 30 mg Injection
- Taxeleon 100 mg Injection
- Taxeleon 260 mg Injection
- Taxeleon 300 mg Injection
- Taxocare 20 mg Injection
- Taxocare 80 mg Injection
- Taxocare 120 mg Injection
- Taxonab 100 mg Injection
- Taxotere 20 mg Injection
- Taxotere 80 mg Injection
- Tecentriq 840 mg Injection
- Tecentriq 1200 mg Injection
- Teceris 0.5 mg Injection
- Tepapro 15 mg Injection
- Tepawel 15 mg Injection
- Tezomib 2 mg Injection
- Themiset Injection
- Thioplan 15 mg Injection
- Thiother 15 mg Injection
- Tinowel 0.5 mg Injection
- Topocan 2.5 mg Injection
- Topocan 4 mg Injection
- Topotec 2.5 mg Injection
- Topotel 2.5 mg Injection
- Torisel Kit
- Toritz 100 mg Injection
- Toritz 500 mg Injection
- Toritz RA 500 mg Injection
- Tortaxel 30 mg Injection
- Tortaxel 100 mg Injection
- Tortaxel 260 mg Injection
- Trabec 1 mg Solution for Infusion
- Trasmab 440 mg Injection
- Trasturel 150 mg Injection
- Trasturel 440 mg Injection
- Trazumab 150 mg Injection
- Trazumab 440 mg Injection
- Trumab 150 mg Injection
- Trumab 440 mg Injection
- Tysabri 300 mg Injection
- Uniblastin 10 mg Injection
- Unicarb 150 mg Injection
- Unicarb 450 mg Injection
- Unicristin 1 mg Injection
- Unifolin 50 mg Injection
- Uniphos 200 mg Injection
- Uniphos 500 mg Injection
- Uniphos 1000 mg Injection
- Uniplatin 10 mg Injection
- Uniplatin 50 mg Injection
- Uromitexan 200 mg Injection
- Vacosteo 5 mg Infusion
- Vectibix 100 mg Injection
- Vegfxta 100 mg Injection
- Vegfxta 400 mg Injection
- Velcade 1 mg Injection
- Velcade 3.5 mg Injection
- Velspan 2 mg Injection
- Veltip 2 mg Injection
- Versavo 100 mg Injection
- Versavo 400 mg Injection
- Vestrant 250 mg Injection
- Vinbicel 50 mg Injection
- Vincreta 1 mg Injection
- Vinelbine 10 mg Injection
- Vinelbine 50 mg Injection
- Vinlon 1 mg Injection
- Vinotec 10 mg Injection
- Viraferonpeg 150 mcg Injection
- Vivitra 150 mg Injection
- Vivitra 440 mg Injection
- Vortuxi 500 mg Injection
- V-strant 250 mg Injection
- Xalipat 50 mg Injection
- Xalipat 100 mg Injection
- Xgeva 120 mg PFS
- Xgrast 300 mcg Injection
- Xmab 100 mg Injection
- Xmab 500 mg Injection
- Xolair 150 mg Injection
- Xphil 300 mcg Injection
- X-Plat 50 mg Injection
- X-Plat 100 mg Injection
- Xpreza 100 mg Injection
- Yervoi 50 mg Injection
- Yondelis Injection
- Zestmab 100 mg Injection
- Zestmab 500 mg Injection
- Zobone 4 mg Injection
- Zodox 10 mg Injection
- Zodox 50 mg Injection
- Zoladex 3.6 mg Injection
- Zoladex LA 10.8 mg Injection
- Zolasta 4 mg Injection
- Zoldonat 4 mg Injection
- Zoldria 4 mg Injection
- Zoledac 4 mg Injection
- Zoleget 4 mg Injection
- Zolephos 5 mg Infusion
- Zolespan 4 mg Injection
- Zolfrac 5 mg Infusion
- Zolistra 4 mg Injection
- Zolshil 4 mg Injection
- Zoltero 4 mg Injection
- Zometa RTU 4 mg Injection
- Zorrent 4 mg Injection
- Zortemib 2 mg Injection
- Zpac 100 mg Injection
- Zpac 260 mg Injection
- Z-Texel Injection
- Zubidox 10 mg Injection
- Zubidox 50 mg Injection
- Zumustin 100 mg Injection
- Zuvicin 50 mg Injection
- Zuvicin 100 mg Injection
- Zybev 100 mg Injection
- Zyclastin 4 mg Injection
- Zynesp 25 mcg PFS
- Zynesp 40 mcg PFS
- Zynesp 100 mcg PFS
- Zyrop 4000 IU Vial
- Zyrop 10000 IU Vial
- Nab Paclitero 100 mg Injection
- Nabcure 100 mg Injection
- Nabpac 100 mg Injection
- Nabtoxol 100 mg Injection
- Nanopac 100 mg Injection
- Nanopacli 100 mg Injection
- Nanoxel 100 mg Injection
- Nanoxel 300 mg Injection
- Adoxi 20 mg Injection
- Adoxi 120mg Injection
- Adriamycin RTU 50 mg Injection
- Albucel 10 gm50 ml Injection
- Albucel 20 gm100 ml Injection
- Alkacel 50 mg Injection
- Alphalan 50 mg Injection
- Aredia 30 mg Injection
- Bdtaxel 100 mg Injection
- Bdtaxel 260 mg Injection
- Bdtaxel 300 mg Injection
- Biobin 1000 mg Injection
- Bioposide 100 mg Injection
- BORTECAD 2MG INJECTION
- Cytarabine 1000 mg Injection
- Oxifect 50 mg Injection
- Octride 0.05 mg Injection
- Neoben 10 mg Injection
- Human Albumin 20 gm/100 ml Injection
- Globucel 10 gm/100 ml Injection
- Globucel 5 gm/100 ml Injection
- Bevacizab 400 mg Injection
- Bevacizab 100 mg Injection
- Pharmaceutical Injection And Solution
- Pharmaceutical Capsules
- Cyclosporine Capsule USP
- Lenalidomide Capsule
- Cyclophil ME - 50 Capsules
- Vingraf 2mg Capsule
- Dutas Capsule
- Spexib 150 Mg Capsule
- Pomalong 1mg Capsule
- Dabigat 150 Capsule
- Glenza 40 Mg Capsule
- Lenangio 10 Mg Capsule
- Netupitant and Palonosetron Hydrochloride capsule
- Isotroin 20 Capsule
- Acozolo 100 mg Capsule
- Acozolo 250 mg Capsule
- Adova 1mg Capsule
- Advagraf 0.5 mg Capsule
- Advagraf 1 mg Capsule
- Advagraf 3 mg Capsule
- Arpimune ME 50 mg Capsule
- Arpimune ME 100 mg Capsule
- Atavir 300 mg Capsule
- Atazor 200 mg Capsule
- Atazor 300 mg Capsule
- Atranza 10 mg Capsule
- Azel 40 mg Capsule
- Azel 80 mg Capsule
- Amitant kit 125 mg /80 mg Capsule
- Apretero 125 mg /80 mg Capsule
- Bdenza 40 mg Capsule
- Bdenza 80 mg Capsule
- Bdfoie 10 mg Capsule
- Bdfoie 4 mg Capsule
- Bdfucil 100 mg + 224 mg Capsule
- Bdpoma 1 mg Capsule
- Bdpoma 2 mg Capsule
- Belustine 40 Capsule
- Ca Atra 10 mg Capsule
- Capmide 40 mg Capsule
- Celomide 5 mg Capsule
- Celomide 10 mg Capsule
- Celomide 25 mg Capsule
- Chemotinib 100 mg Capsule
- Cresemba 100 mg Capsule
- Crizalk 250 mg Capsule
- Cyendiv 100 mg Capsule
- Cyendiv 150 mg Capsule
- Cytodrox 500 mg Capsule
- Dimegen 120 mg Capsule
- dimegen 240 mg capsule
- Dinex EC 250 mg Capsule
- Distamine 500 mg Capsule
- Du kinib 250 mg Capsule
- Durea 500 mg Capsule
- Efavir 200 mg Capsule
- Ematra Capsule
- Emend Capsule
- Encrpc 40 mg Capsule
- Enzamide 40 mg Capsule
- Enzamide 80 mg Capsule
- Enzana 40 mg Capsule
- Enzuta 40 mg Capsule
- Enzyl 40 mg Capsule
- Enzyl 80 mg Capsule
- Estiva 600 Capsule
- Etoplast 50 mg Capsule
- Etosid 50 mg Capsule
- Etovel 50 mg Capsule
- Fluvir 75 mg Capsule
- Gbmt 20 mg Capsule
- Gbmt 100 mg Capsule
- Gbmt 250 mg Capsule
- Glenvas 4 mg Capsule
- Glenza 40 mg Capsule
- Glenza 80 mg Capsule
- Gliotem 20 mg Capsule
- Gliotem 100 mg Capsule
- Gliotem 250 mg Capsule
- Glioz 20 mg Capsule
- Glioz 100 mg Capsule
- Glioz 250 mg Capsule
- Glistroma 100 mg Capsule
- Glistroma 250 mg Capsule
- Grafotas 25 mg Capsule
- Grafotas 50 mg Capsule
- Grafotas 100 mg Capsule
- Nindanib 100 mg Capsule
- Nindanib 150 mg Capsule
- Nindev 100 mg Capsule
- Nintena 150 mg Capsule
- Nintib 100 mg Capsule
- Hodpro 50 mg Capsule
- Hydab 500 mg Capsule
- Hydrogem 500 mg Capsule
- Hydrosar 500 mg Capsule
- Ibatkin 100 mg Capsule
- Ibatkin 400 mg Capsule
- Ibipolid 1 mg Capsule
- Ibrukem 140 mg Capsule
- Nitib 140 mg Capsule
- Ibrumed 140 mg Capsule
- Ibrunat 140 mg Capsule
- Ibrushil 140 mg Capsule
- Ib-tib 140 mg Capsule
- Nitnib 12.5 mg Capsule
- Idofnib 100 mg Capsule
- Nitnib 25 mg Capsule
- Nitnib 50 mg Capsule
- Imbruvica 140 mg Capsule
- Imunotac 0.5 mg Capsule
- Imunotac 1 mg Capsule
- Indivan 400 mg Capsule
- Induz 2.5 mg Capsule
- Nublast 100 mg Capsule
- Nublast 20 mg Capsule
- Nublast 250 mg Capsule
- Lastet 50 mg Capsule
- Laviat 10 mg Capsule
- Lenalid 5 mg Capsule
- Lenalid 10 mg Capsule
- Lenalid 15 mg Capsule
- Lenalid 25 mg Capsule
- Lenangio 5 mg Capsule
- Obnyx 40 mg Capsule
- Lenangio 10 mg Capsule
- Lenangio 25 mg Capsule
- Lenated 10 mg Capsule
- Lenated 25 mg Capsule
- Lenava 10 mg Capsule
- Lenced 4 mg Capsule
- Lenced 10 mg Capsule
- Lendomy 5 mg Capsule
- Lendomy 10 mg Capsule
- Lendomy 25 mg Capsule
- Lenid 5 mg Capsule
- Lenid 10 mg Capsule
- Lenmid 5 mg Capsule
- Lenmid 10 mg Capsule
- Lenmid 25 mg Capsule
- Lenofect 5 mg Capsule
- Lenofect 10 mg Capsule
- Lenofect 25 mg Capsule
- Lenome 5 mg Capsule
- Lenome 10 mg Capsule
- Lenome 25 mg Capsule
- Lenomust 10 Capsule
- Lenshil 4 mg Capsule
- Lenshil 10 mg Capsule
- Lentib 4 mg Capsule
- Lentykine 4 mg Capsule
- Lentykine 10 mg Capsule
- Lenva 4 mg Capsule
- Lenva 10 mg Capsule
- Lenvat 4 mg Capsule
- Lenvat 10 mg Capsule
- Lenvat 10 mg Capsule 30N
- Lenvatol 4 mg Capsule
- Lenvatol 10 mg Capsule
- Lenvenib 4 mg Capsule
- Lenvenib 10 mg Capsule
- Lenvima 4 mg Capsule
- Lenvima 10 mg Capsule
- Lenzest 25 mg Capsule
- Lomoother 40 mg Capsule
- Lomtin 40 mg Capsule
- Luporal Capsule
- Lynide 5 mg Capsule
- Lynide 10 mg Capsule
- Lynide 25 mg Capsule
- Odventa 0.5 mg Capsule
- Mitant 12.5 mg Capsule
- Mitant 25 mg Capsule
- Mitant 50 mg Capsule
- Odventa 1 mg Capsule
- Moostin 40 mg Capsule
- Movfor 200mg Capsule
- Myelostat 500 mg Capsule
- Mylostat 500 mg Capsule
- Mythal 50 mg Capsule
- Mythal 100 mg Capsule
- Palbace 75 mg Capsule
- Palbace 100 mg Capsule
- Palbace 125 mg Capsule
- Palbo 125 mg Capsule
- Oncothal 100 mg Capsule
- Opilo 125 mg /80 mg Capsule
- P Carzine 50 mg Capsule
- Piptashil 125 mg 80 mg Capsule
- Poma 2 mg Capsule
- Poma 4 mg Capsule
- Pomacel 2 mg Capsule
- Pomacel 4 mg Capsule
- Pomahope 3 mg Capsule
- Pomahope 4 mg Capsule
- Pomalex 1 mg Capsule
- Pomalid 1 mg Capsule
- Pomalid 2 mg Capsule
- Pomalid 4 mg Capsule
- Pomalong 1 mg Capsule
- Pomalong 2 mg Capsule
- Pomalong 4 mg Capsule
- Pomavia 2 mg Capsule
- Pomavia 4 mg Capsule
- Pomcad 2 mg Capsule
- Pomide 1 mg Capsule
- Pomide 2 mg Capsule
- Pomide 4 mg Capsule
- Pomired 1 mg Capsule
- Pomired 2 mg Capsule
- Pomired 4 mg Capsule
- Pomyelo 1 mg Capsule
- Pomyelo 2 mg Capsule
- Pomyelo 4 mg Capsule
- Posid 50 mg Capsule
- Rafinlar 50 mg Capsule
- Rcnet 12.5 mg Capsule
- Rcnet 25 mg Capsule
- Rcnet 50 mg Capsule
- Rebetol 200 mg Capsule
- Relidomide 10 mg Capsule
- Ribapro 200 mg Capsule
- Ribasure Capsule
- Ribavin 200 mg Capsule
- Riborea 500 mg Capsule
- Rinhib 200 mg Capsule
- Rolitrans 1 mg Capsule
- Samenza Capsule
- Samluta 40 mg Capsule
- Spexib 150 mg Capsule
- Stavir 30 mg Capsule
- Stavir 40 mg Capsule
- Sunisam 12.5 mg Capsule
- Sunisam 25 mg Capsule
- Sunisam 50 mg Capsule
- Sunishil 12.5 mg Capsule
- Sunishil 25 mg Capsule
- Sunishil 50 mg Capsule
- Sunitax 12.5 mg Capsule
- Sunitax 25 mg Capsule
- Sunixar 12.5 mg Capsule
- Sunixar 25 mg Capsule
- Sutent 12.5 mg Capsule
- Sutent 25 mg Capsule
- Sutent 50 mg Capsule
- Sutib 12.5 mg Capsule
- Sutib 25 mg Capsule
- Sutib 50 mg Capsule
- Sutinat 12.5 mg Capsule
- Sutinat 25 mg Capsule
- Sutinat 50 mg Capsule
- Sutykine 12.5 mg Capsule
- Sutykine 25 mg Capsule
- Tacrograf 0.5 mg Capsule
- Tacrograf 1 mg Capsule
- Tacromus 0.5 mg Capsule
- Tacromus 0.25 mg Capsule
- Tacromus 1 mg Capsule
- Tacromus 2 mg Capsule
- Takfa 0.5 mg Capsule
- Takfa 0.25 mg Capsule
- Takfa 1 mg Capsule
- Takfa 2 mg Capsule
- Tamiflu 75 mg Capsule
- Tasigna 150 mg Capsule
- Tasigna 200 mg Capsule
- Tauritmo 25 mg Capsule
- Tegafi Capsule
- Tegura Capsule
- Temcad 20 mg Capsule
- Temcad 100 mg Capsule
- Temcad 250 mg Capsule
- Temodal 20 mg Capsule
- Temodal 100 mg Capsule
- Temodal 250 mg Capsule
- Temoget 20 mg Capsule
- Temoget 250 mg Capsule
- Temolon 100 mg Capsule
- Temolon 250 mg Capsule
- Temonat 20 mg Capsule
- Temonat 250 mg Capsule
- Temorel 20 mg Capsule
- Temorel 100 mg Capsule
- Temorel 140 mg Capsule
- Temorel 250 mg Capsule
- Temoside 20 mg Capsule
- Temoside 100 mg Capsule
- Temoside 250 mg Capsule
- Temospan 100 mg Capsule
- Temospan 250 mg Capsule
- Temotero 20 mg Capsule
- Temotero 100 mg Capsule
- Temotero 250 mg Capsule
- Temother 20 mg Capsule
- Temother 100 mg Capsule
- Temotide 250 mg Capsule
- Temozorix 100 mg Capsule
- Temright 20 mg Capsule
- Temright 100 mg Capsule
- Temright 250 mg Capsule
- Terigen 14 mg Capsule
- Thaangio 50 mg Capsule
- Thalide 100 mg Capsule
- Thalitero 50 mg Capsule
- Thalitero 100 mg Capsule
- Thalix 50 mg Capsule
- Thalix 100 mg Capsule
- Thaloda 50 mg Capsule
- Thaloda 100 mg Capsule
- Thaloma 100 mg Capsule
- Thycad 50 mg Capsule
- Thycad 100 mg Capsule
- Tiloshil 250mg Capsule
- Timinib 400 mg Capsule
- Tybriva 140 mg Capsule
- Unidrea 500 mg Capsule
- Unitinib 400 mg Capsule
- Uracel 100 mg224 mg Capsule
- Uratuf 100 mg 224 mg Capsule
- Veenat 100 mg Capsule
- Viranz 600 mg Capsule
- Virazide 100 mg Capsule
- Virofix 200 mg Capsule
- X trant 100 mg Capsule
- Xtandi 40 mg Capsule
- Xylutide 40 mg Capsule
- Zinocarb 50 mg Capsule
- Zymmune 25 mg Capsule
- Zymmune 50 mg Capsule
- Zymmune 100 mg Capsule
- Alecensa 150 mg Capsule
- Amitant kit 125 mg 80 mg Capsule
- Aprecap Capsule
- Apretero 125 mg 80 mg Capsule
- Bdfucil 100 mg + 224 mg Capsule
- Palbace 100mg Capsule
- Bdfucil 100 mg + 224 mg Capsule
- Bdfucil 100 mg + 224 mg Capsule
- Pharmaceutical Tablets
- Pomalid 4mg Capsules
- Abamune Tablet
- Sevcar 800 Tablet
- Valgan Tablet
- Tacrolimus Capsules
- Valganciclovir Tablets
- Entecavir Tablets
- Voriconazole Tablets
- Azathioprine USP 50mg Tablet
- Ketosteril Tablet
- Picasa-GR Tablet
- Aplazar Tablets
- Gutron 2.5mg Tablet
- Duta-steride Capsules
- Uric Acid Tablet
- Revelol XL 25 Tablet
- Pirfenex Tablet
- Hepcinat Plus 60MG/400MG Tablet
- Acivir 400 Dt Tablet
- Methylprednisolone Tablets Ip 4 Mg
- Methylprednisolone Tablets 16 Mg
- Demson Tablets 8mg
- Merislon 6 Tablet
- Dexam 4mg Tablet
- Virolfi 450mg Tablet
- Mofetyl-S 360 Tablet
- Nodosis - DS Tablet
- Lenmid 25mg Tablet
- Prednisone 10mg tablet
- Prednisone 20 Mg Tablet
- Abalam Tablet
- Abatitor 250 mg Tablet
- Abec L Tablet
- Abec 300 mg Tablet
- Abione 250 mg Tablet
- Abirapro 250 mg Tablet
- Abiratas 250 mg Tablet
- Abirapro 500 mg Tablet
- Abiracure 250 mg Tablet
- Abiratas 500 mg Tablet
- Abiratred 500 mg Tablet
- Abitate 250 mg Tablet
- Abitate U 250 mg Tablet
- Ablu 50 mg Tablet
- Abnib 250 mg Tablet
- Abpacli 100 mg Injection
- Abretone 250 mg Tablet
- Abstet 250 mg Tablet
- Acogenib 250 mg Tablet
- Aconast 1 mg Tablet
- Acutrol 400 mg Tablet
- Acutrol 800 Tablet
- Acutrol C 400 mg Tablet
- Acutrol C 800 mg Tablet
- Adesera 10 mg Tablet
- Adfovir 10 mg Tablet
- Adlante 4 mg Capsule
- Advacan 0.5 mg Tablet
- Afanat 20 mg Tablet
- Afanat 30 mg Tablet
- Afanat 40 mg Tablet
- Afinitor 5 mg Tablet
- Ahabir 250 mg Tablet
- Albavir Tablet
- Alentos 0.5 mg Tablet
- Alkacel 5 mg Tablet
- Alkeprost 250 mg Tablet
- Alkeprost 500 mg Tablet
- Alkeran 2 mg Tablet
- Alphalan 2 mg Tablet
- Alphalan 5 mg Tablet
- Alsatinib 100 mg Tablet
- Aromita 1mg Tablet
- Arospan 1 mg Tablet
- Alsatinib 20 mg Tablet
- Alsatinib 50 mg Tablet
- Altanib NOVA 400 mg Tablet
- Asunra 400 mg Tablet
- Atazor R Tablet
- Atraz 1 mg Tablet
- Aluvia 200 mg/50 mg Tablet
- Avantaz 1 mg Tablet
- Avigan 200 mg Tablet
- Avobi 50 mg Tablet
- Avonza Tablet
- Axishil 1 mg Tablet
- Axishil 5mg Tablet
- Axpero 1 mg Tablet
- Axpero 5 mg Tablet
- Axzyb 1 mg Tablet
- Axzyb 5 mg Tablet
- Azadine O Tablet
- Azofit 50 mg Tablet
- Azoran 50 mg Tablet
- Azoran 75 mg Tablet
- Anabrez 1 mg Tablet
- Anacan 1 mg Tablet
- Anaday 1 mg Tablet
- Anaglob 1 mg Tablet
- Anamit 1mg Tablet
- Anaridex 1 mg Tablet
- Anarzole 1 mg Tablet
- Anashil 1 mg Tablet
- Anastar 1 mg Tablet
- Anastrocare 1 mg Tablet
- Anastron 1 mg Tablet
- Anastronat 1 mg Tablet
- Anatero 1 mg Tablet
- Anazol 1 mg Tablet
- Androblok 50 mg Tablet
- Anosam 1 mg Tablet
- Arbitus 250 mg Tablet
- Aritoz 1 mg Tablet
- Armilon 1 mg Tablet
- Armotraz 1 mg Tablet
- Bandrone 150 Tablet
- Bandrone 50 mg Tablet
- Baraclude 0.5 mg Tablet
- Baraclude 1 mg Tablet
- Bdaxit 1 mg Tablet
- Bdcyclo 50 mg Tablet
- Bd-letro 2.5 mg Tablet
- Bdocin 500 mg Tablet
- Bdparib 200 mg Tablet
- Bdparib 300 mg Tablet
- Bdpoma 4 mg Tablet
- Bdron 500 mg Tablet
- Bonitar 100 MG TABLET
- BONITAR 400 MG TABLET
- Beedan 100 mg Tablet
- BONRISE 150MG TABLET
- Beedan 100 mg Tablet
- Beedan 20 mg Tablet
- Beedan 50 mg Tablet
- Bosutris 100 mg Tablet
- Bosutris 400 mg Tablet
- Bosutris 500 mg Tablet
- Bosuvi 100 mg Tablet
- Bosuvi 400 mg Tablet
- Bosuvi 500 mg Tablet
- Brestozol 1mg Tablet
- Bretolap 250 mg Tablet
- Brutrax 1 mg Tablet
- Bucelon 2 mg Tablet
- Busulmax 2 mg Tablet
- Cabolong 20mg Tablet
- Cabotib 40 mg Tablet
- Cabotib 60 mg Tablet
- Cacit 500 mg Tablet
- Cadilap 250 mg Tablet
- Caditam 10 mg Tablet
- Caditam 20 mg Tablet
- Caditraz 2.5 mg Tablet
- Cadsonib 200 mg Tablet
- Caluran 50 CP Tablet
- Calured 50 mg Tablet
- Calutide 50 mg Tablet
- Capcel 500 mg Tablet
- Capecite 500 mg Tablet
- Capegard 500 mg Tablet
- Capetero 500 mg Tablet
- Capezam 500 mg Tablet
- Capget 500 mg Tablet
- Capiibine 500 mg Tablet
- Capnat 500 mg Tablet
- Capsy 500 mg Tablet
- Captomer 50 mg Tablet
- Bexinib 5 mg Tablet
- Bic 50 mg Tablet
- Bical 50 mg Tablet
- Bicalon 50 mg Tablet
- Bicalpro 50 mg Tablet
- Bicatero 50 mg Tablet
- Casodex 50 mg Tablet
- Cassotide 50 mg Tablet
- Castramid Tablet
- Celkeran 2 mg Tablet
- Celkeran 5 mg Tablet
- Cellcept 250 mg Tablet
- Cellcept 500 mg Tablet
- Cellmune 500 mg Tablet
- Celofem 2.5 mg Tablet
- Celonib 100 mg Tablet
- Celonib 400 mg Tablet
- Celstrol 160 mg Tablet
- Certican 0.5 mg Tablet
- Certican 0.25 mg Tablet
- Certican 0.75 mg Tablet
- Chemlet 2.5 mg Tablet
- Chemofit 250 mg Tablet
- Chemogef 250 mg Tablet
- Chemotinib 400 mg Tablet
- Chloramax 5 mg Tablet
- Chloramax 2 mg Tablet
- Cimivir 400 mg Tablet
- Cimivir-L Tablet
- Cipmido 2.5 mg Tablet
- Citabin 500 mg Tablet
- Clokeran 2 mg Tablet
- Clokeran 5 mg Tablet
- Clomipure-L Tablet
- Combinib 250 mg Tablet
- Combivir 150 mg300 mg Tablet
- Comcapsy 400 mg20 mg Tablet
- Comcapsy 700 mg30 mg Tablet
- Cosalon 50 mg Tablet
- Cronivir 0.5 mg Tablet
- Cycloxan 50 mg Tablet
- Cytomid 250 mg Tablet
- Cytotam 10 mg Tablet
- Cytotam 20 Tablet
- Dacihep 30 Tablet
- Dacihep 60 mg Tablet
- Biovorin 15 mg Tablet
- Daclacruz 60 mg Tablet
- Daclacure 60 mg Tablet
- Daclahep 60 mg Tablet
- Daclawin 60 mg Tablet
- Dacoplice 15 mg Tablet
- Dacoplice 30 mg Tablet
- Dacoplice 45 mg Tablet
- Dalsiclear 60 mg Tablet
- Daruvir 600 mg Tablet
- Daruvir 800 mg Tablet
- Dasafav 20 mg Tablet
- Dasafav 50 mg Tablet
- Dasafav 100 mg Tablet
- Dasamyl 50 mg Tablet
- Dasamyl 70 mg Tablet
- Dasanat 20 mg Tablet
- Dasanat 50 mg Tablet
- Dasania 50 mg Tablet
- Dasapan 50 mg Tablet
- Dasapan 70 mg Tablet
- Dasashil 20 mg Tablet
- Dasashil 50 mg Tablet
- Dasashil 70 mg Tablet
- Dasashil 100 mg Tablet
- Dasatrue 50 mg Tablet
- Dasatrue 70 mg Tablet
- Daslemia 50 mg Tablet
- Daslemia 70 mg Tablet
- Daslemia 100 mg Tablet
- Dastila 50 mg Tablet
- Defrijet 250 mg Tablet
- Defrijet 500 mg Tablet
- Desifer 100 mg Tablet
- Desifer 400 mg Tablet
- Dinmek Tablet
- Natdac 60 mg Tablet
- Duovir Tablet
- Duovir N Tablet
- Duovir-E Kit
- Durart-R 450 Tablet
- Durataf 25 mg Tablet
- Dyronib 20 mg Tablet
- Dyronib 50 mg Tablet
- Dyronib 70 mg Tablet
- Efamat 600 mg Tablet
- Efavir 600 Tablet
- Efcure 200 mg Tablet
- Efcure 600 mg Tablet
- Effoday 300 mg300 mg600 mg Tablet
- Egsure 1 mg Tablet
- Elinal 1 mg Tablet
- Emduo 150 mg30 mg Tablet
- Emletra Tablet
- Empetus 100 mg Tablet
- Emtaf-B Tablet
- Encure Tablet
- Endace 40 mg Tablet
- Endace 160 mg Tablet
- Endoxan 50 mg Tablet
- Entaliv 0.5 Tablet
- Entavir 0.5 mg Tablet
- Entavir 1 mg Tablet
- Entax 10 mg Tablet
- Entax 20 mg Tablet
- Zavedos 5 mg Capsule
- ENTECA 0.5 mg Tablet
- Entehep 0.5 Tablet
- Entehep 1 Tablet
- E-Ova L 2.5 Tablet
- Erleva 100 mg Tablet
- Erleva 150 mg Tablet
- Erlocip 100 mg Tablet
- Erlocip 150 mg Tablet
- Erlonat 100mg Tablet
- Erlonat 150 mg Tablet
- Erlot 100 mg Tablet
- Erlot 150 mg Tablet
- Erlotero 100 mg Tablet
- Erlotero 150 mg Tablet
- Erlotib 100 mg Tablet
- Erlotib 150 mg Tablet
- Etibo 250 mg Tablet
- Evadiol 2 mg Tablet
- Everbliss 5 mg Tablet
- Everbliss 10 mg Tablet
- Evercan 10 mg Tablet
- Evergraf 0.5 mg Tablet
- Evergraf 0.25 mg Tablet
- Evermil 5 mg Tablet
- Evermil 10 mg Tablet
- Everomus 0.5 mg Tablet
- Everomus 0.25 mg Tablet
- Everotas 0.5 mg Tablet
- Everotas 0.25 mg Tablet
- Evertor 5 mg Tablet
- Evertor 10 mg Tablet
- Exeget 25 mg Tablet
- Exetraz 25 mg Tablet
- Femara 2.5 mg Tablet
- Femistra 1 mg Tablet
- Femitraz 1 mg Tablet
- Fempro 2.5 mg Tablet
- Fineova 40 Tablet
- Fludabine 10 mg Tablet
- Flutamide 250 mg Tablet
- Flutatec 250 mg Tablet
- Flutide 250 mg Tablet
- Nectki 100 mg Tablet
- Neivex 250 mg Tablet
- Forstavir EM Tablet
- Neostrol 160 mg Tablet
- Neostrol 40 mg Tablet
- Geffon 250 mg Tablet
- Geffy 250 mg Tablet
- Gefitec 250 mg Tablet
- Gefitero Tablet
- Gefitrust Tablet
- Neovorin 15 mg Tablet
- Gefonib 250 mg Tablet
- Geftib 250 mg Tablet
- Gefticip 250 mg Tablet
- Geftilon 250 mg Tablet
- Geftinat 250 mg tablet
- Geftistar 250 mg Tablet
- Geftiwel 250 mg Tablet
- Nevilast 30 Tablet
- Nevilast 40 Tablet
- Nevir ER Tablet
- Nevir Tablet
- Nexavar 200 mg Tablet
- Nextki 50 mg Tablet
- Nextki 70 mg Tablet
- Glivec 100 mg Tablet
- Glivec 400 mg Tablet
- G-nib 250 mg Tablet
- Goodova-L 5 mg Tablet
- Goodova-L Tablet
- Gutron 2.5 mg Tablet
- Hepalo 0.5 mg Tablet
- Hepbest 25 mg Tablet
- Hepcdac 60 mg Tablet
- Hepcfix 60 mg Tablet
- Hepcinat Plus Tablet
- Hepcinat Tablet
- Hepcinat-LP Tablet
- Hepcvel Tablet
- Hepcvir 400 mg Tablet
- Hepcvir L Tablet
- Heptavir 100 mg Tablet
- Heptavir 150 mg Tablet
- Heptral 400 mg Tablet
- Herduo 250 mg Tablet 30N
- Herlapsa 250 mg Tablet
- Hertab 250 mg Tablet 30N
- Hertab 250 mg Tablet 150N
- Hivus-LR 50 mg200 mg Tablet
- Hycamtin 1 mg Tablet
- Ibawin 150 mg Tablet
- Idromet 50 mg Tablet
- Nolvadex 10 mg Tablet
- Imaget 400 mg Tablet
- Imalek 100 mg Tablet
- Novisof 400 mg Tablet
- Imalek 400 mg Tablet
- Imanex 400mg Tablet
- Imanib 100 mg Tablet
- Imanib 400 mg Tablet
- Imat 100 mg Tablet
- Imat 400 mg Tablet
- Imated 400 mg Tablet
- Imatero 100 mg Tablet
- Imatero 400 mg Tablet
- Imatib 100 mg Tablet
- Imatib 400 mg Tablet
- Imatirel 400 mg Tablet
- Noxafil Tablet
- Inbec Tablet
- Inlyta 1 mg Tablet
- Inlyta 5 mg Tablet
- Innomune 50 mg Tablet
- Instgra Tablet 30N
- Instgra Tablet 90N
- Intajac 5 mg Tablet
- Nuace Tablet
- Invista 50 mg Tablet
- Invista 70 mg Tablet
- Invista 100 mg Tablet
- Iressa 250 mg Tablet
- Jakavi 5 mg Tablet
- Jakavi 15 mg Tablet
- Jakavi 20 mg Tablet
- Jaknat 5 mg Tablet
- Nublexa 40 mg Tablet
- Jakura 5 mg Tablet
- Kryxana 200 mg Tablet
- Kytib 1 mg Tablet
- Kytib 5 mg Tablet
- Lamihope 150 mg Tablet
- Lamivir 150 mg Tablet
- Lamivir HBV Tablet
- Lanib 250 mg Tablet
- Lanolimus 0.5 mg Tablet
- Lanolimus 0.25 mg Tablet
- Lanvis 40 mg Tablet
- Lapahope 250 mg Tablet 30N
- Lapahope 250 mg Tablet 120N
- Lapatem 250 mg Tablet
- Nuparp 200 mg Tablet
- Nuparp 300 mg Tablet
- Lavir 150 mg Tablet
- Lazid E Kit
- Lazid N Tablet
- Lazid Tablet
- Ledifos Tablet
- Ledihep Tablet
- Ledviclear Tablet
- Lefumide 10 mg Tablet
- Lefumide 20 mg Tablet
- Lesovir Tablet
- Letall 2.5 mg Tablet
- Letero 2.5 mg Tablet
- Letoval Tablet
- Letrofil 2.5 mg Tablet
- Letrohope 2.5 Tablet
- Letronat 2.5 mg Tablet
- Letroz 2.5 mg Tablet
- Leucorin 15 mg Tablet
- Levin 400 mg Tablet
- Libset 250 mg Tablet
- Lopimune Tablet
- Lupinib 400 mg Tablet
- Mamazol 2.5 mg Tablet
- Maximune 500 mg Tablet
- Megahenz 40 mg Tablet
- Megahenz 160 mg Tablet
- Megasty 160 mg Tablet
- Megeetron 40 mg Tablet
- Megeetron 160 mg Tablet
- Megetra 40 mg Tablet
- Megetra 160 mg Tablet
- Melfalax 2 mg Tablet
- Melfalax 5 mg Tablet
- Meqsel 0.5 mg Tablet
- Mercapto 50 mg Tablet
- Mhega 160 mg Tablet
- Mitinab 400 mg Tablet
- Oleptiss 360 mg Tablet
- Mofetyl 250 mg Tablet
- Mofetyl 500 mg Tablet
- Mofigen 500 mg Tablet
- Mycofit 250 mg Tablet
- Mycofit 500 mg Tablet
- Mycofit S 180 mg Tablet
- Mycofit S 360 mg Tablet
- Mycomune 500 mg Tablet
- Mycomune S 360 mg Tablet
- Mydacla 60 mg Tablet
- Myhb 2 mg Tablet
- Myhb 4 mg Tablet
- Myhep 400 mg Tablet
- Myhep All Tablet
- Myhep Dvir Tablet
- Myhep Lvir Tablet
- Mytera 250 mg Tablet
- Mytera 500 mg Tablet
- Mytinib 400 mg Tablet
- Oleptiss 360 mg Tablet
- Olumiant 2 mg Tablet
- Olumiant 4 mg Tablet
- Oncolet 2.5 mg Tablet
- Osral 60 mg Tablet
- Picasa GR Tablet
- Piclib 125 mg Tablet
- Pivikto 150 mg Tablet
- Pivikto 200 mg Tablet
- Posatral Tablet
- Posoxil GR Tablet
- Pro-5 500 mg Tablet
- Prograf 0.5 mg Tablet
- Prograf 1 mg Tablet
- PTH 30 mg Tablet
- Purinethol 50 mg Tablet
- Qubol Tablet
- Qvir Kit
- Ralista Tablet
- Ralofen 60 mg Tablet
- Ramiven 50 mg Tablet
- Ramiven 100 mg Tablet
- Ramiven 150 mg Tablet
- Ramiven 200 mg Tablet
- Rapacan 1 mg Tablet
- Rapact 5 mg Tablet
- Rapact 10 mg Tablet
- Rapasim 1 mg Tablet
- Reatix Kit
- Reclaim-L Tablet
- Regonat 40 mg Tablet
- Relicitabine 500 mg Tablet
- Relimidex 1 mg Tablet
- Renchek Tablet
- Renostrong Tablet
- Renvela 800 mg Tablet
- Resihance 40 mg Tablet
- Resof Total Tablet
- Resof-L Tablet
- Revenz 600 mg Tablet
- Reviro 300 mg Tablet
- Revolade 25 mg Tablet
- Revolade 50 mg Tablet
- Ribahep Capsule
- Ricovir 300 mg Tablet
- Ricovir EM Tablet
- Ritocom Tablet
- Ritomune 100 mg Tablet
- Ritovaz 300 mg100 mg Tablet
- Rocas 1 mg Tablet
- Rolimus 5 mg Tablet
- Rolimus 10 mg Tablet
- Samitib 400 mg Tablet
- Samtica 250 mg Tablet
- Samtide 50 mg Tablet
- Sebivo 600 mg Tablet
- Siromus 1 mg Tablet
- Siropan 1 mg Tablet
- Sirotrend 1 mg Tablet
- Sirova 1 mg Tablet
- Sofab 400 mg Tablet
- Sofab LP Tablet
- Sofocruz LP Tablet
- Sofocure L Tablet
- Sofocure Tablet
- Sofocure V Tablet
- Sofokem L Tablet
- Sofokem Tablet
- Sofovir Tablet
- Sonora 200 mg Tablet 30N
- Sonora 200 mg Tablet 120N
- Sorafenat 200 mg Tablet
- Soranib Tablet
- Soratib 200 mg Tablet
- Sovihep 400 mg Tablet
- Sovihep V Tablet
- Spegra Tablet 30N
- Spegra Tablet 90N
- Spnib 50 mg Tablet
- Spnib 100 mg Tablet
- Stadin 30 mg Tablet
- Stadin 40 mg Tablet
- Stazonex 1 mg Tablet
- Stimufol 2.5 mg Tablet
- Stimu-Let 2.5 mg Tablet
- Stimu-Let 5 mg Tablet
- Stritinib 400 mg Tablet
- Synthivan Tablet
- T Jaki 5 mg Tablet
- Tabi 50 mg Tablet
- Tafero EM Tablet
- Tafero Tablet
- Tafnat Tablet
- Tafsure 25 mg Tablet
- Tagrisso 80 mg Tablet
- Tamodex 10 mg Tablet
- Tamoxilon 10 mg Tablet
- Tamoxilon 20 mg Tablet
- Tamtero 10 mg Tablet
- Tamtero 20 mg Tablet
- Tarceva 100 mg Tablet
- Tarceva 150 mg Tablet
- Tavin EM Tablet
- Tavin L Tablet
- Tavin Tablet
- Teevir Tablet
- Telura Tablet
- Tenfoclear 300 mg Tablet
- Tenfoplus 25 mg Tablet
- Tenocruz 300 mg Tablet
- Tenof 300 mg Tablet
- Tenof EM Tablet
- Tenohep AF Tablet
- Tenohep Tablet
- Tenolam E Tablet
- Tenolam-AR Kit
- Tentide AF 25 mg Tablet
- Tentide EM Tablet
- Tenvir 300 mg Tablet
- Tenvir AF 25 mg Tablet
- Tenvir EM Tablet
- Teravir 300 mg Tablet
- Tofajak 5 mg Tablet
- Tofe 5 mg Tablet
- Trioday Tablet
- Triomune 150 mg30 mg200 mg Tablet
- Trivir 30 Tablet
- Trivir 40 Tablet
- Trombopag 25 mg Tablet
- Trombopag 50 mg Tablet
- Trustiva Tablet
- Truvada Tablet
- Tykerb 250 mg Tablet
- Tylidys 250 mg Tablet
- Tyrokinin 150 mg Tablet
- Unifolin 15 mg Tablet
- Unistrol 40 mg Tablet
- Utamide Tablet
- Valodex 10 mg Tablet
- Valodex 20 mg Tablet
- Valten 300 mg Tablet
- Vebalone 150 mg Tablet
- Veenat 400 mg Tablet
- Velasof Tablet
- Velpaclear Tablet
- Velpacruz S 400 mg100 mg Tablet
- Velpanat Tablet
- Vianca Tablet 30N
- Vianca Tablet 90N
- Viraday Tablet
- Virataz R 300 mg100 mg Tablet
- Virbetol Tablet
- Viread 300 mg Tablet
- Virem 300 mg Tablet
- Virem 600 mg Tablet
- Virem R Tablet
- Viro 4 Kit
- Viroclear 400 mg Tablet
- Virocomb 150 mg300 mg Tablet
- Virol 300 mg Tablet
- Viropil Tablet 30N
- Viropil Tablet 90N
- Virotrenz Tablet
- Virpas Tablet
- Virso Tablet
- Volantis 5 mg Tablet
- Volantis 10 mg Tablet
- Vonaday Tablet
- Vonavir Tablet
- Votrient 200 mg Tablet
- Votrient 400 mg Tablet
- Womazol Tablet
- X Vir 0.5 mg Tablet
- X Vir 1 mg Tablet
- Xarelto 10 mg Tablet
- Xarelto 20 mg Tablet
- Xbira 250 mg Tablet
- Xbira 500 mg Tablet
- Xcel 25 mg Tablet
- Xecap 500 mg Tablet
- Xefta 250 mg Tablet
- Xeljanz 5 mg Tablet
- Xeloda 500 mg Tablet
- Xenosoul Tablet
- Xmalon 25 mg Tablet
- Xolimus 10 mg Tablet
- Xovoltib 20 mg Tablet
- Xovoltib 30 mg Tablet
- Xovoltib 40 mg Tablet
- Xovoltib 50 mg Tablet
- Xtane 25 mg Tablet
- Zelgor 250 mg Tablet
- Zelgor 500 mg Tablet
- Zepdon 400 mg Tablet
- Ziddivir 100 mg Tablet
- Ziddivir 300 mg Tablet
- Zidine 300 mg Tablet
- Zidolam 150 mg300 mg Tablet
- Zidolam N N 150 mg300 mg200 mg Tablet
- Zidovir 100 mg Capsule
- Zidovir 300 mg Tablet
- Zovilam 150 mg300 mg Tablet
- Zufinib 250 mg Tablet
- zuviphos 50 mg
- Zyceva 100 mg Tablet
- Zyceva 150 mg Tablet
- Zymurine 50 mg Tablet
- Zytiga 250 mg Tablet
- Abamune L Tablet
- Abec L Tablet
- Abec Tablet
- Advacan 0.25 mg Tablet
- Afinitor 10 mg Tablet
- Alkacel 2 mg Tablet
- Altraz 1mg Tablet
- Altrol 1 mg Tablet
- Aluvia 200 mg50 mg Tablet
- Aromasin 25 mg Tablet
- Axishil 5 mg Tablet
- Bdaxit 5 mg Tablet
- Bdron 250 mg Tablet
- Bexinib 1 mg Tablet
- Erlonat 100 mg Tablet
- Hivus-LR 50 mg/200 mg Tablet
- Effoday 300 mg/300 mg/600 mg Tablet
- Comcapsy 700 mg/30 mg Tablet
- Comcapsy 400 mg/20 mg Tablet
- Combivir 150 mg/300 mg Tablet
- Bonrise 150 mg Tablet
- Beedan 70 mg Tablet
- Arimidex 1 mg Tablet
- Anti Diabetic Drug
- Cardiac Medicines
- Eye Drops
- Dermatology Products
- Pharmaceutical Vaccines
- Anti Hiv Drug
- Duovir N Tablet
- Tenofovir Disoproxil Fumarate Tablets
- Efavirenz 600mg Tablets
- Tenvir 300mg Tablets
- Spegra Tablets
- Wepox 10000 Iu Injection
- Emtri Tablet
- Viro 4 Kit
- Aluvia 200mg/50mg
- Atazor R Tablets
- Trioday Tablets
- Trustiva Tablet
- Efavir 200mg Tablet
- Nevimune 200mg Tablet
- Daruvir 600 Mg Tablets
- Cymgal 450 Mg Tablet
- Viraday Tablet
- Pharmaceutical Products
- Isoflurane USP 30ml
- Sevorane 250ml
- Sevorane 250ml
- Isoflurane 100ml
- Isoflurane 250ml Piramal
- Pharmaceutical Products
- Aprepitant Capsule
- Aldara 5% cream
- Apretero 125/80 Kit
- Bonmax pth autopen
- BONMAX PTH CARTRIDAGE 3 ML
- Estogel 80 gm Cream
- Exelon Patch 5 (4.6 mg) Patch
- Exelon Patch 10 (9.5 mg) Patch
- Exelon Patch 15 (13.3 mg) Patch
- Fluvir 12mgml Suspension
- Nirmacom Combipack
- Menactra Vaccine 0.5 ml
- Influvac Tetra 20232024 Vaccine
- Mabura 40 mg Pen
- Memory Touch 9 mg patch
- Memory Touch 18 mg patch
- Olimab 60 mg PFS
- Pneumovax 23 Vaccine 0.5ml
- Prevenar 13 Vaccine 0.5 ml
- Renvela 800 mg sachets
- Rowasa 1 gm sachet
- Rowasa 500 mg sachet
- Shanchol Shanchol
- Stamaril vaccine
- Terifrac Multi(Pen Device)
- Terifrac Solo Prefilled Pen
- Typbar PFS
- Zymmune Oral Suspension
- Fluvir 12mg/ml Suspension
- Abbott Free Style Optium H Strips
- Accuchek Aviva
- Antibiotic Tablets
- Pharmaceutical Medicine
- Vaccines
- Sevoflurane
- Anti Cancer Tablets
- Azel 40 Mg Capsule
- Capsy 500 Mg Tablet
- Demson Tablets 8mg
- Hertraz 440mg Injection
- Imanib 400 Mg Tablet
- Imatirel 400 MG Tablet
- Myelostat 500 Mg Capsule
- Riborea 500 Mg Capsules
- Tamodex 10 Mg Tablets
- Trasturel 440 Mg Injection
- Vivitra 440mg Injection
- X Trant 140 Mg Capsule
- Xtane 25 Mg Tablet
- Fempro Tablet
- Leucorin 15 Mg Tablet
- Cytomid 250 Tablets
- Endace 40 Tablet
- Herlapsa Tablet
- Cacit 500mg Tablet
- Mamofen 20 Tablet
- Gefitinib Tablets IP 250 mg
- Xbira 250mg Tablet
- Zyceva 100mg Tablet
- Zecyte 250mg Tablet
- Megetra 160mg Tablet
- Tykerb 250 Mg Tablet
- Geffy 250 Mg Tablet
- Femistra Tablet
- Erlonat 150 Tablet
- Erlocip 150 Tablets
- Ramiven 200mg Tablet
- Abiraterone Acetate
- Cyclophosphamide 50mg tablet
- Everolimus 10mg tablet
- Alkeran 2mg Tablet
- Pivikto 150mg Tablet
- Apaluside 60MG Tablet
- Arimidex 1mg Tablet
- Aromasin 25mg Tablet
- Scemblix 40mg tablet
- Avapritinib Tablet 200 Mg
- Bdaxit 5mg Tablet
- Ayvakit Avapritinib 100mg Tablets
- Balversa Erdafitinib Tablets
- Belzutifan 40 mg Tablet
- Sorafenat 200mg Tablet
- Veenat 400 Tablet
- Hiv Medicines
- Antifungal medicine
- Antiviral medicine
- Anticancer medicine
- Bevacirel 400mg Injection
- Difidox Doxycycline 100mg Injection
- Oncology Drug
- Bevatas 400 Solution for Infusion
- Cytax 260 Injection
- Oxitoz 100mg Injection
- Thalix 50 Capsules
- Lupride Injection
- Glioz 20mg Capsule
- Bortenat 3.5mg Injection
- Celginase 10000IU Injection
- Celdach 100 Mg Injection
- Dacotin 100 Infusion
- Epithra 10 Mg Injections
- Daunotec 20 Mg Injection
- Cyramza 500 Mg Injection
- Paclitaxel Abraxane 100mg Injection
- Adriamycin 50mg Injection
- BLEOCIP 15MG INJECTION
- Vincristine 1mg
- Etoposide injection
- Paclitaxel 100mg injection
- Docetaxel 120mg injection
- Taxotere 80mg Injection
- Tocilizumab 162mg injection
- Brentuximab Vedotin 50mg injection
- Cytarabine injection
- Cytarabine 500mg injection
- Ado-Trastuzumab Emtansine 100mg injection
- Afatinib Dimaleate 40mg tablet
- Pomalid 2mg Capsule
- Lemtrada 12mg Infusion
- Campath Alemtuzumab 30 mg/ml Injection
- Alkeran 50 Mg Injection
- Rybrevant (amivantamab-vmjw) Injection
- Arimidex 1mg Tablet
- Aredia 30mg Injection
- Arranon Nelarabine Injection
- Arsenic Trioxide 10mg injection
- Asparlas (calaspargase pegol-mknl) Injection
- Bavencio Injection
- Azadine Injection
- Bleocel 15IU Injection
- Posid 100mg Injection
- Posid 100mg Capsule
- CYCLOXan 1000mg Injection
- P Carzine 50mg Capsule
- Blenrep belantamab Injection
- Beleudaq 500 mg Injection
- Purplz 100mg Injection
- Bendit Bendamustine Hydrochloride Injection
- Bleomycin Sulphate Injection
- Celplat Cisplatin 50Mg Platinol injection
- Immunosuppressant
- Analgesic
- Anti Coagulants
- ANTI CANCER INJECTION
- Iron Replacement Injection
- Anti Biotic Injection
- Contact Us

Bevatas 400 Solution for Infusion
39600.0 INR/Pack
Product Details:
- Drug Type General Medicines
- Physical Form Liquid
- Recommended For Doctor
- Dosage Guidelines As per Instructions
- Suitable For Adults
- Storage Instructions Cool & Dry Place
- Click to view more
X
Bevatas 400 Solution for Infusion Price And Quantity
- 1 Pack
- 39600.0 INR/Pack
Bevatas 400 Solution for Infusion Product Specifications
- Adults
- Doctor
- Cool & Dry Place
- Liquid
- General Medicines
- As per Instructions
Bevatas 400 Solution for Infusion Trade Information
- Cash Advance (CA), Cash in Advance (CID), Telegraphic Transfer (T/T)
- 100 Pack Per Week
- 6 Days
- No
- If order is confirmed we will reimburse the sample cost
- Eastern Europe, Middle East, Africa, Western Europe, Asia, Australia, Central America, North America, South America
- All India
Product Description
1. Technical Specifications
| Attribute | Details |
|---|---|
| Brand Name | Bevatas 400 Solution for Infusion |
| Active Ingredient | Bevacizumab (recombinant humanized monoclonal antibody; anti-VEGF) |
| Strength | 400 mg/16 mL (25 mg/mL) |
| Dosage Form | Concentrate for solution for IV infusion (must be diluted) |
| Packaging Size | 1 single-use vial |
| Packaging Type | Type I glass vial with rubber stopper in carton |
| Medicine Type | Allopathic, Antineoplastic (Targeted biologic; anti-angiogenic) |
| Manufactured By | Intas Pharmaceuticals Ltd. |
| Distributed By | Authorized oncology distributors / hospital pharmacies |
2. Product Description
Bevatas (bevacizumab) is an anti-angiogenic monoclonal antibody that binds VEGF-A, preventing activation of VEGF receptors (VEGFR-1/2). This inhibits tumor blood-vessel formation, starving tumors of oxygen and nutrients and enhancing the effects of chemotherapy. It is for IV infusion only and is used across multiple solid tumors under oncologist supervision.
3. Product Highlights
| Attribute | Details |
|---|---|
| Generic Name | Bevacizumab |
| Therapeutic Category | Targeted therapy / Anti-VEGF monoclonal antibody |
| Administration | IV infusion after dilution in 0.9% NaCl (never IV push/bolus) |
| Typical Dosing | 515 mg/kg IV every 23 weeks (indication-specific) |
| First Infusion Rate | 90 min (if tolerated, next infusions may be 60 then 30 min) |
| Diluent | 0.9% sodium chloride only (do not use dextrose) |
| Storage | 28 C, do not freeze or shake, protect from light; single-use vialdiscard unused portion |
4. Therapeutic Uses
-
Metastatic colorectal cancer (with 5-FUbased chemotherapy)
-
Non-squamous NSCLC (with platinum-based chemotherapy; not for squamous histology due to bleeding risk)
-
Epithelial ovarian, fallopian tube, primary peritoneal cancer (front-line and recurrent settings in combinations/maintenance as per protocol)
-
Cervical cancer (persistent/recurrent/metastatic, with chemotherapy)
-
Renal cell carcinoma (with interferon alfa or per protocol)
-
Glioblastoma (recurrent, per local guidelines)
-
Hepatocellular carcinoma (with atezolizumab, per protocol)
Exact indication and regimen should follow current local labels/guidelines.
5. Side Effects
Very common/common:
-
Hypertension
-
Proteinuria (may be asymptomatic; can progress to nephrotic syndrome)
-
Fatigue, headache
-
Diarrhea, stomatitis, appetite loss
-
Bleeding events (e.g., epistaxis), mucocutaneous hemorrhage
-
Delayed wound healing
-
Infusion-related reactions
Serious/clinically significant:
-
Gastrointestinal perforation, fistula formation
-
Severe hemorrhage (pulmonary, GI, CNScan be fatal)
-
Arterial and venous thromboembolism (TIA/stroke/MI, DVT/PE)
-
Congestive heart failure (risk with prior anthracyclines/radiation)
-
Posterior reversible encephalopathy syndrome (PRES/RPLS)
-
Severe hypertension/hypertensive crisis
-
Severe proteinuria/nephrotic syndrome
-
Wound-healing complications
-
Gastrointestinal anastomotic complications (post-op)
6. Precautions
-
Boxed/critical warnings: GI perforation, severe hemorrhage, and impaired wound healing.
-
Blood pressure: Control before starting; monitor regularly; treat per guidelines; withhold for severe/uncontrolled hypertension.
-
Proteinuria: Check urine protein periodically; withhold/discontinue for 2 g/24 h or nephrotic syndrome per protocol.
-
Surgery/wounds: Stop 28 days before elective surgery and resume 28 days after and only when wounds are fully healed.
-
Bleeding risk: Avoid in patients with recent significant hemoptysis or high bleeding risk; not indicated for squamous NSCLC.
-
Arterial/venous thromboembolism: Use caution in patients with risk factors; discontinue for serious events.
-
Cardiac function: Monitor in patients with prior anthracyclines/chest irradiation or cardiac history.
-
Pregnancy & fertility: Embryo-fetal toxicity; use effective contraception during treatment and for at least 6 months after the last dose. May impair female fertility (ovarian failure). Avoid breastfeeding during therapy and for 6 months after last dose.
-
Hypersensitivity: Contraindicated in patients with known serious hypersensitivity to bevacizumab or CHO-cell products.
Precision Dosage for Effective Results
Bevatas 400 Solution for Infusion is carefully administered by healthcare professionals in strict accordance with prescribed dosage instructions. This ensures that patients receive the optimal therapeutic benefit while minimizing potential risks or side effects.
Specialized Distribution and Reliability
The product is sourced and supplied by trusted distributors, exporters, suppliers, traders, and wholesalers across India. Each unit is handled to maintain quality and safety, ensuring patients receive genuine, effective medication.
FAQs of Bevatas 400 Solution for Infusion:
Q: How should Bevatas 400 Solution for Infusion be administered?
A: Bevatas 400 Solution for Infusion must be administered intravenously by qualified healthcare professionals, following a doctors prescription and dosage instructions. Self-administration is not recommended.Q: What is the recommended dosage for Bevatas 400 Solution for Infusion?
A: The dosage of Bevatas 400 is strictly determined by a physician based on individual patient needs and medical conditions. Always follow the doctors instructions and do not alter the dosage without consultation.Q: When is this medication most commonly used?
A: Bevatas 400 is used when prescribed by a doctor for specific medical conditions. It is administered as part of a treatment plan for adults and not for general or over-the-counter usage.Q: Where should Bevatas 400 Solution for Infusion be stored?
A: This medication should be stored in a cool and dry place, away from direct sunlight and moisture, to retain its efficacy and safety.Q: What is the process for obtaining Bevatas 400 Solution for Infusion in India?
A: Licensed distributors, exporters, suppliers, traders, and wholesalers across India provide this product. It is only available with a valid doctors prescription through authorized channels.Q: Is Bevatas 400 Solution suitable for self-use or self-prescription?
A: No, Bevatas 400 Solution is strictly for use under medical supervision and should only be used as prescribed by a qualified doctor. It is not intended for self-medication.Q: What are the potential benefits of using Bevatas 400 Solution for Infusion?
A: When used appropriately under a doctors guidance, Bevatas 400 Solution can offer significant therapeutic benefits for adults requiring targeted medical intervention as part of their prescribed treatment plan.Tell us about your requirement

Price:
Quantity
Select Unit
- 50
- 100
- 200
- 250
- 500
- 1000+
Additional detail
Mobile number
Email



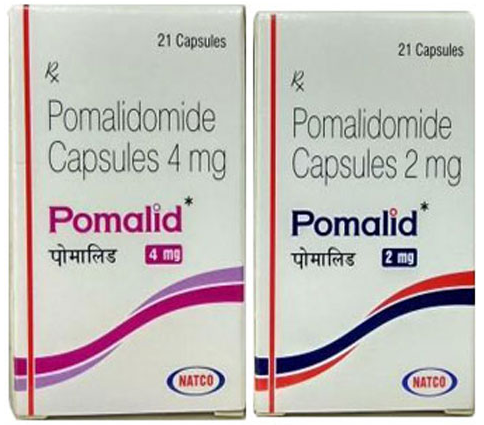
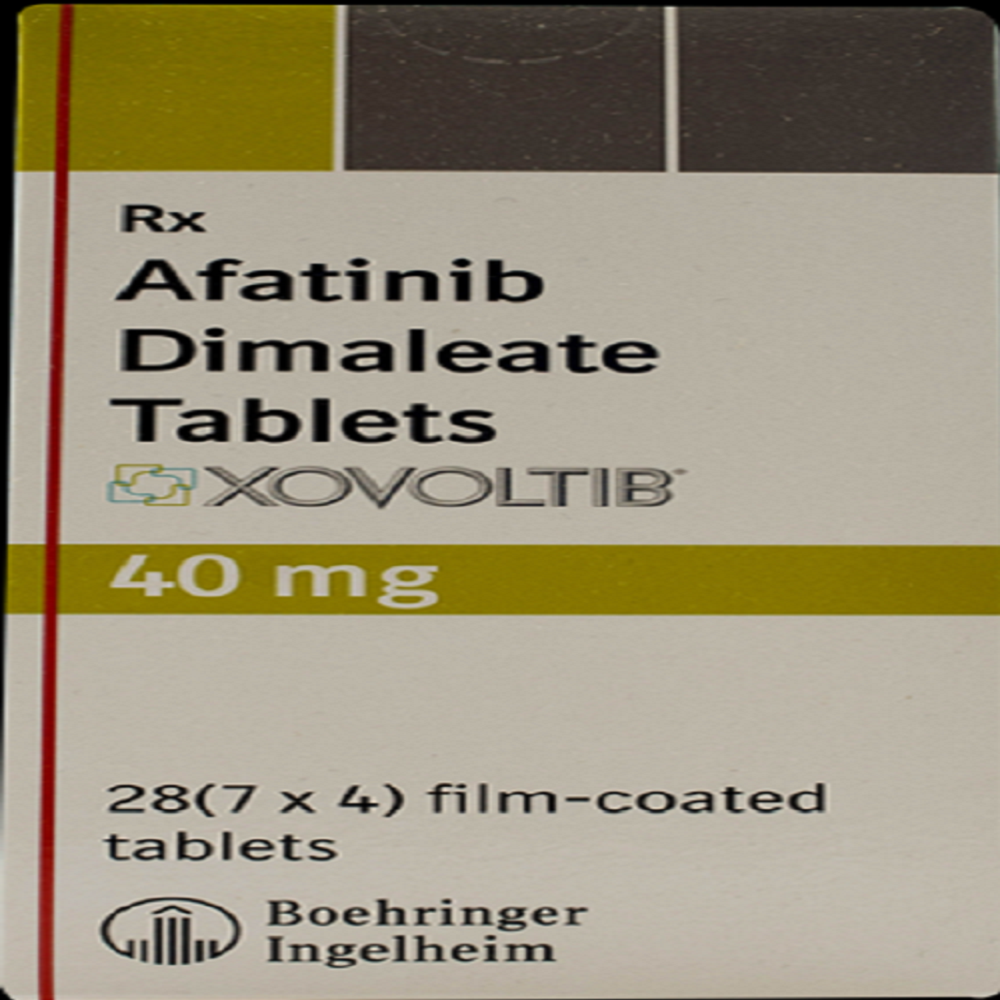
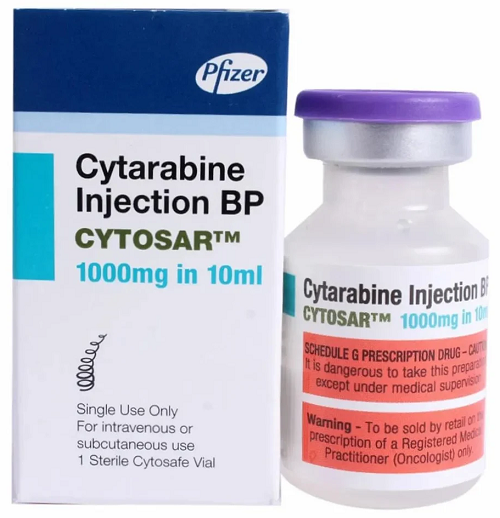

 English
English Spanish
Spanish French
French German
German Italian
Italian Chinese (Simplified)
Chinese (Simplified) Japanese
Japanese Korean
Korean Arabic
Arabic Portuguese
Portuguese