- होम पेज
- कंपनी प्रोफाइल
-
हमारे उत्पाद
- टीबी की दवाएँ
- एंटीबायोटिक दवाएँ
- फार्मास्युटिकल इंजेक्शन
- फिल्ग्रास्टिम इंजेक्शन
- हयालूरोनिडेज़ इंजेक्शन
- त्वचीय भराव
- सेफोपेराज़ोन सल्बैक्टम इंजेक्शन
- कार्बोप्लेटिन इंजेक्शन
- पेगास्टा इंजेक्शन
- एरिथ्रोपोइटिन अल्फ़ा इंजेक्शन
- फिल्ग्रास्टिम इंजेक्शन पीएफएस 300 एमसीजी
- टेरिपैराटाइड इंजेक्शन
- एरिथ्रोपोइटिन इंजेक्शन
- एपिरूबा 50एमजी इंजेक्शन
- ज़ायरोप 4000IU इंजेक्शन
- एन्सिकार्ब इंजेक्शन 100mg/2ml
- विंटोर
- फार्मास्युटिकल इंजेक्शन और समाधान
- फार्मास्युटिकल कैप्सूल
- फार्मास्युटिकल गोलियाँ
- मधुमेह विरोधी दवा
- हृदय संबंधी औषधियाँ
- आंखों में डालने की बूंदें
- त्वचाविज्ञान उत्पाद
- फार्मास्युटिकल टीके
- एचआईवी विरोधी दवा
- दवा उत्पाद
- एबॉट फ्री स्टाइल ऑप्टियम एच स्ट्रिप्स
- एक्यूचेक अवीवा
- एंटीबायोटिक गोलियाँ
- औषधि चिकित्सा
- टीके
- सेवोफ़्लुरेन
- संपर्क करें
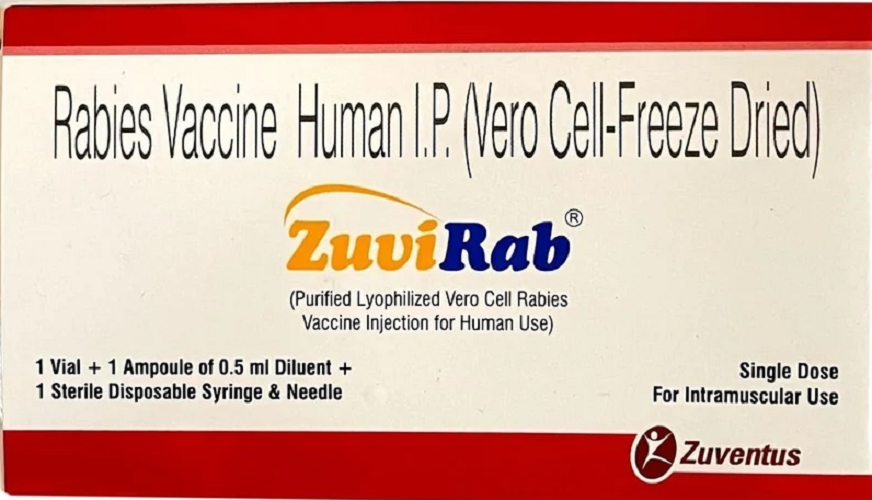
Zuvirab Vaccine
643.0 आईएनआर/Pack
उत्पाद विवरण:
- खुराक As directed by physician
- संकेत For active immunization against rabies
- स्टोरेज निर्देश Dry Place
- फॉर्मूलेशन टाइप Injection
- प्रॉडक्ट टाइप Vaccine
- फ़ीचर
- उपचार और कार्य
- Click to view more
X
मूल्य और मात्रा
- 40
उत्पाद की विशेषताएं
- 1 ml
- वर्ष
- For active immunization against rabies
- As directed by physician
- Dry Place
- Injection
- Vaccine
व्यापार सूचना
- , ,
- प्रति सप्ताह
- दिन
- No
- , , , , , , , ,
उत्पाद विवरण
1. Technical Specifications
| Attribute | Details |
|---|---|
| Brand Name | Zuvirab |
| Generic Name | Rabies Human Monoclonal Antibody / Rabies Vaccine (depending on formulation) |
| Strength | 0.5 ml per vial (single dose) |
| Dosage Form | Injection (suspension for intramuscular use) |
| Packaging Size | Single vial (0.5 ml) |
| Manufacturer | Zydus Cadila Healthcare Ltd. |
| Therapeutic Class | Immunobiological / Vaccine |
| Medicine Type | Allopathic |
2. Product Description
Zuvirab 0.5 ml Injection is a purified inactivated rabies vaccine used for both pre-exposure prophylaxis and post-exposure prophylaxis of rabies infection.
It stimulates the bodys immune system to produce antibodies against the rabies virus, thereby preventing the disease after suspected exposure (e.g., dog bites, scratches, or contact with potentially rabid animals).
3. Product Highlights
| Attribute | Details |
|---|---|
| Indication | Prevention of rabies infection |
| Application Area | Pre-exposure immunization, Post-exposure treatment |
| Administration | Intramuscular injection (deltoid region in adults, anterolateral thigh in children) |
| Schedule | As per WHO & National Guidelines usually multiple doses required for full protection |
| Storage Conditions | Store at 28 C (Refrigerated). Do not freeze. |
4. Therapeutic Uses
-
Pre-exposure prophylaxis: For individuals at high risk (veterinarians, animal handlers, lab workers, travelers to endemic areas).
-
Post-exposure prophylaxis: After suspected rabies exposure (animal bites, scratches, or contact with saliva).
-
Administered along with Rabies Immunoglobulin (RIG) in category III exposures (severe bites).
5. Side Effects
Common:
-
Pain, redness, or swelling at injection site
-
Mild fever
-
Headache, dizziness
-
Fatigue, malaise
Rare but serious:
-
Severe allergic reaction (anaphylaxis)
-
Neurological reactions (extremely rare)
6. Precautions
-
Should only be given under medical supervision.
-
Must not be injected into the gluteal region (reduced effectiveness).
-
Contraindicated in individuals with severe allergic reaction to previous rabies vaccination or any vaccine component.
-
Pregnancy and breastfeeding: can be used when risk of rabies exposure is high (benefit outweighs risk).
-
Immunocompromised patients may require additional doses or monitoring for antibody response.
Comprehensive Rabies Protection
Zuvirab provides robust immunization against rabies, offering both pre-exposure and post-exposure prophylaxis. Its formulation meets global safety and efficacy standards and is suitable for diverse age groups, making it ideal for use in hospitals, clinics, and personal healthcare strategies.
Advanced Lyophilized Formulation
Zuvirab features a lyophilized rabies vaccine derived from human diploid cell cultures. This formulation extends shelf life and ensures stability when stored in dry conditions, preserving potency and effectiveness during transportation and storage.
FAQs of Zuvirab Vaccine:
Q: How is Zuvirab administered for rabies immunization?
A: Zuvirab is administered intramuscularly, typically in the deltoid area, by a healthcare professional. The exact dosage and schedule will be determined by your physician depending on whether you require pre-exposure or post-exposure prophylaxis.Q: What are the applications and benefits of Zuvirab vaccine?
A: Zuvirab is used for active immunization against rabies in both pre-exposure and post-exposure scenarios. Its timely administration provides essential protection against rabies virus infection, supporting personal health and public safety.Q: When should Zuvirab be used after potential rabies exposure?
A: Zuvirab should be administered as soon as possible following potential exposure to rabies. Early initiation increases the chances of preventing the onset of the disease and is crucial for effective post-exposure management.Q: Where can I receive Zuvirab vaccination?
A: Zuvirab is available at hospitals, clinics, and healthcare centers across India. It must be administered by trained medical professionals under medical supervision as a prescription medication.Q: What process is involved in preparing and storing Zuvirab vaccine?
A: Zuvirab is supplied as a lyophilized (freeze-dried) vial, which is reconstituted before injection. It should be stored in a dry place as per manufacturers guidelines to maintain its shelf life and efficacy.Q: Is Zuvirab suitable for all age groups?
A: Yes, Zuvirab is formulated to provide rabies vaccination for both children and adults. Dosage and schedule are adjusted as directed by a physician based on age, health status, and risk factors.Tell us about your requirement

Price: Â
Quantity
Select Unit
- 50
- 100
- 200
- 250
- 500
- 1000+
Additional detail
मोबाइल number
Email


 मुझे निःशुल्क कॉल करें
मुझे निःशुल्क कॉल करें
 English
English Spanish
Spanish French
French German
German Italian
Italian Chinese (Simplified)
Chinese (Simplified) Japanese
Japanese Korean
Korean Arabic
Arabic Portuguese
Portuguese