- होम पेज
- कंपनी प्रोफाइल
-
हमारे उत्पाद
- टीबी की दवाएँ
- एंटीबायोटिक दवाएँ
- फार्मास्युटिकल इंजेक्शन
- फिल्ग्रास्टिम इंजेक्शन
- हयालूरोनिडेज़ इंजेक्शन
- त्वचीय भराव
- सेफोपेराज़ोन सल्बैक्टम इंजेक्शन
- कार्बोप्लेटिन इंजेक्शन
- पेगास्टा इंजेक्शन
- एरिथ्रोपोइटिन अल्फ़ा इंजेक्शन
- फिल्ग्रास्टिम इंजेक्शन पीएफएस 300 एमसीजी
- टेरिपैराटाइड इंजेक्शन
- एरिथ्रोपोइटिन इंजेक्शन
- एपिरूबा 50एमजी इंजेक्शन
- ज़ायरोप 4000IU इंजेक्शन
- एन्सिकार्ब इंजेक्शन 100mg/2ml
- विंटोर
- फार्मास्युटिकल इंजेक्शन और समाधान
- फार्मास्युटिकल कैप्सूल
- फार्मास्युटिकल गोलियाँ
- मधुमेह विरोधी दवा
- हृदय संबंधी औषधियाँ
- आंखों में डालने की बूंदें
- त्वचाविज्ञान उत्पाद
- फार्मास्युटिकल टीके
- एचआईवी विरोधी दवा
- दवा उत्पाद
- एबॉट फ्री स्टाइल ऑप्टियम एच स्ट्रिप्स
- एक्यूचेक अवीवा
- एंटीबायोटिक गोलियाँ
- औषधि चिकित्सा
- टीके
- सेवोफ़्लुरेन
- संपर्क करें
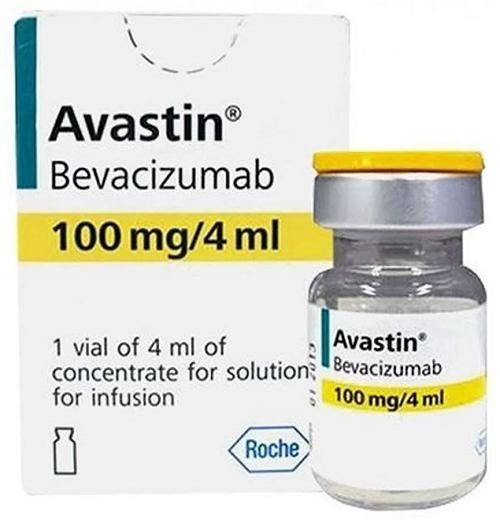
Avastin 100mg Injection
27087.0 आईएनआर/Pack
उत्पाद विवरण:
- दवा का प्रकार
- भौतिक रूप
- के लिए सुझाया गया Doctor
- खुराक संबंधी दिशा-निर्देश As per Instructions
- के लिए उपयुक्त
- स्टोरेज निर्देश Cool & Dry Place
- Click to view more
X
मूल्य और मात्रा
- 8
उत्पाद की विशेषताएं
- Cool & Dry Place
- As per Instructions
- Doctor
व्यापार सूचना
- , ,
- प्रति सप्ताह
- दिन
- No
- , , , , , , , ,
उत्पाद विवरण
1. Technical Specifications
| Attribute | Details |
|---|---|
| Brand Name | Avastin 100mg Injection |
| Generic Name | Bevacizumab |
| Strength | 100 mg / 4 ml (25 mg/ml) |
| Dosage Form | Injection (concentrate for solution for infusion) |
| Packaging Size | Single-use vial (100 mg/4 ml) |
| Packaging Type | Glass vial with rubber stopper & flip-off cap |
| Medicine Type | Targeted Anticancer Therapy (Monoclonal Antibody, VEGF inhibitor) |
| Manufacturer | Roche Products India Pvt. Ltd. / Genentech (a member of Roche Group) |
| Distributed By | Authorized oncology distributors / specialty pharmacies |
2. Product Description
Avastin 100mg Injection contains Bevacizumab, a recombinant humanized monoclonal antibody that specifically binds to vascular endothelial growth factor (VEGF-A).
By inhibiting VEGF-A, Avastin prevents the growth of new blood vessels (angiogenesis) that tumors need for oxygen and nutrients, thereby suppressing tumor growth and metastasis.
It is used in multiple advanced cancers, often in combination with chemotherapy.
3. Product Highlights
| Attribute | Details |
|---|---|
| Therapeutic Category | Targeted Anticancer Monoclonal Antibody (Anti-VEGF) |
| Application Area | Colorectal cancer, NSCLC, Renal cell carcinoma, Glioblastoma, Cervical cancer, Ovarian cancer |
| Mechanism of Action | Binds VEGF-A inhibits angiogenesis reduces tumor blood supply inhibits tumor growth |
| Administration | Intravenous infusion (not IV push/bolus); given over 3090 minutes, dose varies per cancer type |
| Storage Conditions | Store at 28C (refrigerated); do not freeze; protect from light; do not shake |
4. Therapeutic Uses
-
Metastatic Colorectal Cancer (with fluoropyrimidine-based chemo)
-
Non-Small Cell Lung Cancer (NSCLC) (non-squamous, advanced/metastatic) with platinum-based chemo
-
Glioblastoma (as single agent in recurrent disease)
-
Metastatic Renal Cell Carcinoma (with interferon alfa)
-
Persistent, Recurrent, or Metastatic Cervical Cancer (with chemo)
-
Ovarian, Fallopian Tube, or Peritoneal Cancer (first-line, relapsed, platinum-sensitive/resistant cases with chemo)
5. Side Effects
Common:
-
Hypertension
-
Fatigue, headache
-
Proteinuria (protein in urine)
-
Nosebleeds, minor bleeding
-
Delayed wound healing
-
Diarrhea, abdominal pain
Serious / Severe:
-
Gastrointestinal perforation (rare but life-threatening)
-
Hemorrhage (serious bleeding)
-
Thromboembolic events (stroke, heart attack)
-
Severe hypertension & hypertensive crisis
-
Nephrotic syndrome due to proteinuria
-
Reversible posterior leukoencephalopathy syndrome (RPLS rare brain condition)
6. Precautions
-
Wound Healing: Do not initiate within 28 days of major surgery; discontinue in case of wound healing complications.
-
Hypertension: Monitor blood pressure regularly; treat as required.
-
Proteinuria: Regular urine protein monitoring advised.
-
Bleeding Risk: Use with caution in patients with recent hemoptysis, bleeding disorders, or anticoagulant therapy.
-
Pregnancy & Breastfeeding: Contraindicated; may harm fetus (VEGF inhibition can impair fetal development).
-
Drug Interactions: Generally low, since it is a monoclonal antibody, but caution with other myelosuppressive/anti-angiogenic drugs.
Tell us about your requirement

Price: Â
Quantity
Select Unit
- 50
- 100
- 200
- 250
- 500
- 1000+
Additional detail
मोबाइल number
Email



 मुझे निःशुल्क कॉल करें
मुझे निःशुल्क कॉल करें
 English
English Spanish
Spanish French
French German
German Italian
Italian Chinese (Simplified)
Chinese (Simplified) Japanese
Japanese Korean
Korean Arabic
Arabic Portuguese
Portuguese